Aldehydes, Ketones and Carboxylic Acids
Properties and Uses of Aldehydes and Ketones
Physical properties :
1. HCHO is a gas with irritating smell. It is sold as 40% aqueous solution (with 8% CH3OH) under the name formalin.
2. Formalin is used as preservative for biological specimen.
3. Acetone has pleasant smell and is used as an excellent organic solvent, nail polish remover etc.
4. C6H5CHO has bitter almond smell. It is also known as oil of bitter almond.
5. C6H5 COCH3 is steam volatile solid
6. HCHO, CH3CHO and CH3COCH3 are 100% soluble in water
7. Melting points and boiling points increases with increase in C - atoms
8. Branching decreases boiling point and melting points but the spherical symmetry of the molecule increases the melting point.
9. Ketones have slightly high m.p than isomeric aldehydes.
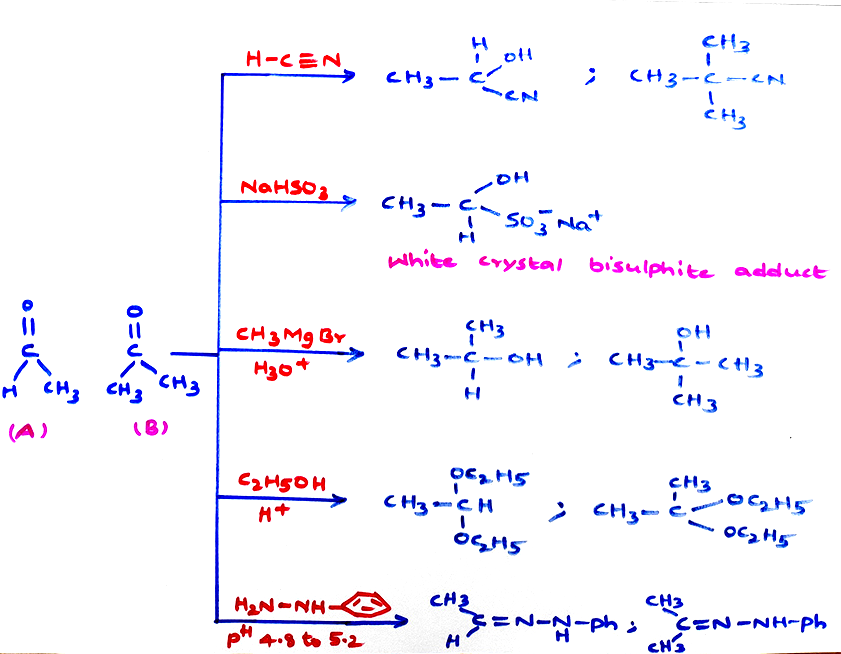
Chemical Properties :
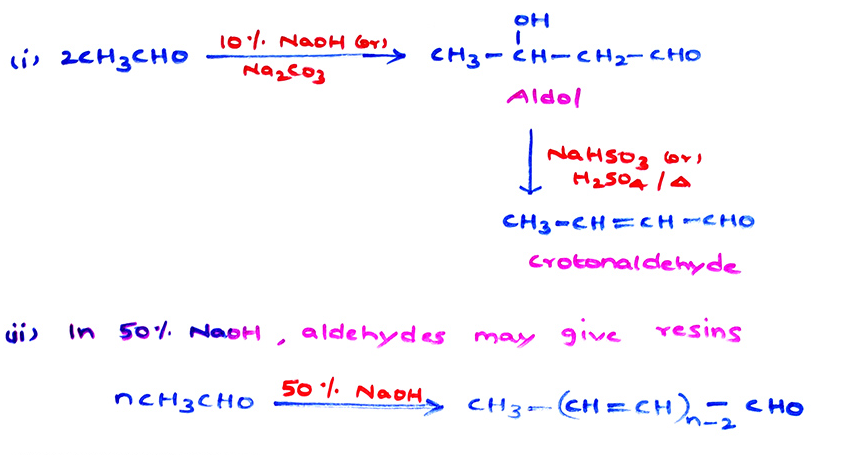
b) Aldol condensation :
Migration of α-H atom from one carbonyl molecule to another, in the presence of dilute (10%) alkali (or) HCl(g) at 10°C gives β-hydroxy carbonyl compound known as aldol which on strong heating eliminates water molecule.
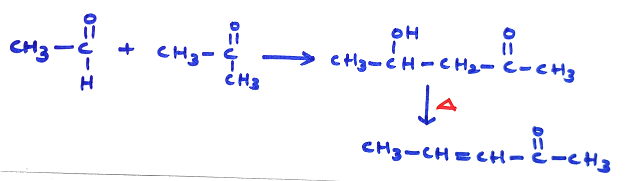
(C) Crossed Aldol Condensation :
(i) Two different aldehydes, both having α-H-atom, produce four products. Major product is formed by migration of more acidic α-H atom.
(ii) For same acidic nature of α-H atom migration occurs to that  group where C-atom has higher positive charge.
group where C-atom has higher positive charge.
(iii) When acetophenone (Hypnone) is heated with C2H5ONa (or) aluminium tert-butoxide DYPNONE is formed.
(iv) If one carbonyl compound has no α-H-atom the reaction is called as Claisen Schmidt (as Claisen reaction).
(v) Intra molecular aldol condensation : It forms five (or) six membered rings.
D) Cannizzaro Reaction : In the presence of 50% aqueous (or) alcoholic NaOH aldehydes having no α-H- atom show auto oxidation and reduction i.e., disproportionation to give alcohol and acid salt at room temperature.

(iii) Simple α-mono alkylated aldehydes having α-H-atom also show cannizzaro reaction at about 300°C. These of course also show aldol condensation
(iv) CCl3CHO does not contain α-H-atom. On heating with 50% aqueous NaOH, it forms CCl3CH2OH and CCl3COONa mainly and a very little of CHCl3
(v) Crossed cannizzaro reaction: when two different aldehydes having no α-H-atom react; the lower one gives its acid salt because of higher positive charge an C-atom 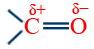 group of lower aldehyde, attack of OH− is easy on Cδ+.
group of lower aldehyde, attack of OH− is easy on Cδ+.
C6H5 − CHO + HCHO + NaOH → C6H5CH2OH + HCOONa
(vii) Internal cannizzaro reaction : It is related to a single molecule having two (or) more CHO groups (better for O - positions and weaker for m - positions) but no α-H-atom
Part1: View the Topic in this Video from 0:25 to 45:25
Part2: View the Topic in this Video from 0:25 to 43:10
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

