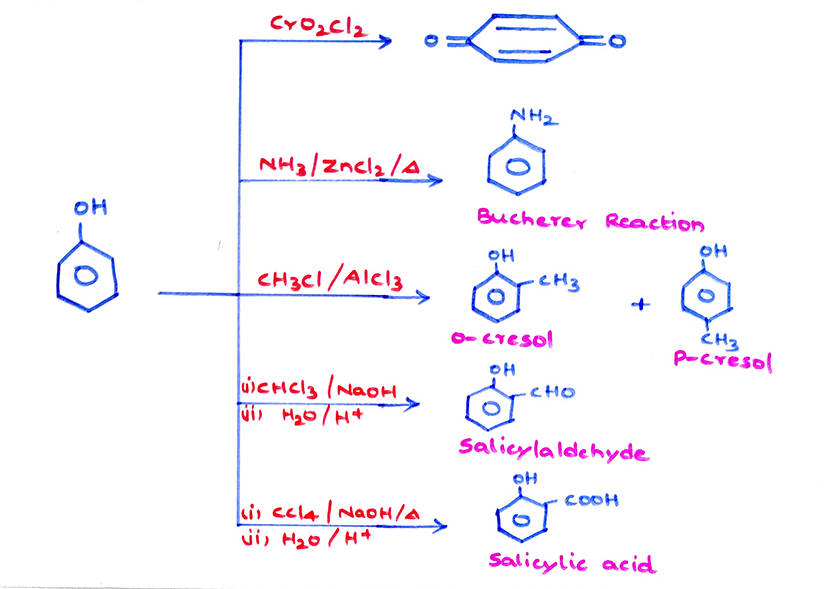
Alcohols, Phenols and Ethers
Properties of Alcohols and Phenols
Some Industrial forms:
(a) 95.6% v/v or 95% w/w ethyl alcohol – H2O mixture is azeotropic mixture.
(b) 90% ethanol + 9% CH3OH + 1% petroleum oil + violet dye → Methylated spirit.
(c) 100% pure C2H5OH is called absolute alcohol.
(d) About 20% ethanol +80% gasoline (v/v) mixture is used in engines of air-crafts. This added alcohol is called power alcohol.
(e) A mixture of C2H5OH, (CH3COO)2Ca and C17H35COOH is solid and is called as solid (fuel) alcohol.
Properties of Alcohols, Phenol and Glycol:
Physical properties:
(a) State: up to C11, alcohols are liquids while higher members are waxy solids.
(b) Toxic nature: Though all alcohols are toxic, methanol is deadly poisonous and can cause blindness and death.
(c) Solubility: Lower members are easily miscible with water while for higher members solubility decreases due to increase in size of hydrophobic "R" part.
solubility of isomeric alcohols increases with increase in branching due to decrease in relative volume of hydrophobic R part 1° < 2° < 3°
(d) Boiling points: Increase in carbon chain increases boiling point by 18-20°C per c-atom.
CH3OH (64.5°C) < C2H5OH (78.3°C) < CH3–CH2–CH2OH (97°C) < CH3CH2–CH2–CH2–CH2OH (118°C)
Chemical properties:
A. Cleavage of O–H bond
Phenols are more acidic than alcohols because the phenol ion is resonance stabilized.
(a) Reaction with Na: All alcohols react with Na to produce H2 gas. 2° alcoholic group of glycerol does not react.
(b) Reaction with NaOH: Only phenol reacts showing it to be most acidic among all these compounds.
(c) Reaction with acids: Ester with fruity smell are produced. Order of reactivity:
(1) CH3OH > 1° > 2° > 3°
(2) HCOOH > CH3COOH >....> R2CH–COOH > R3C–COOH
(i) In the presence of H2SO4, as catalyst, 67% completion of reaction takes place. 'OH' of acid goes to give water.
(ii) In the presence of HCl gas, the reaction, Fischer-spier reaction, goes to approximately 100% completion.
\tt R-OH+R'COOH\xrightarrow{HCl_{(g)}}R'COOR+H_2O
(iii) Acid chloride and acid anhydride also produce esters. The reaction of benzoyl chloride in the presence of dil NaOH is called Schotten Baumann reaction.
(a) Heating of phenyl benzoate in the presence of anhydrous AlCl3, produces ketones. The rearrangement is called
Fries rearrangement.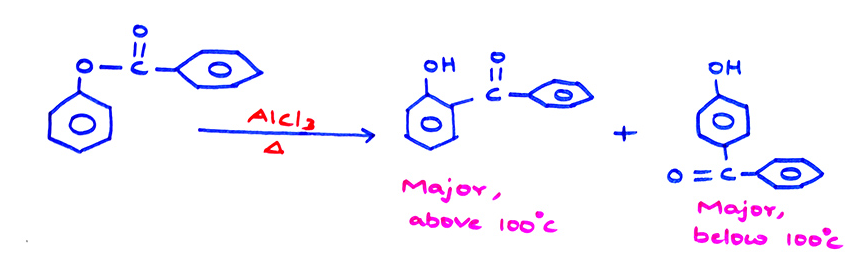
(iv) Reaction of ethylene glycol with p-phthalic acid or its dimethyl ester, followed by polymerisation gives polyester, terylene while o-phthalic acid, the product is glyptal (used in paints and varnishes).
(d) \tt ROH(or\ ArOH)\xrightarrow[HBF_4]{CH_2N_2}(R/Ar)-O-CH_3
B. Cleavage of C-O Bond:
(a) \tt R-OH_{(g)}+NH_{3(g)}\xrightarrow[633K]{Al_2O_3(or)ThO_2}R-NH_2+R_2NH+R_3N(Industrial\ method)
(b) \tt C_6H_5OH+NH_3\xrightarrow[ZnCl_2/press]{573K}C_6H_5NH_2(Aniline)+H_2O(Bucherer\ reaction)
(c) \tt C_6H_5OH\xrightarrow[\Delta]{Zn(dust)}C_6H_6(Benzene)+ZnO(Reduction\ reaction)
(d)(i) Reaction of HX (order HI > HBr > HCl), HCl/ZnCl2, PCl5, PCl3, SOCl2 produce corresponding halogen compounds.
(ii) The reactions of PCl5 and SOCl2 (Darzen process, best for 1° alcohol) are SN1 reaction. No rearrangement take place in the absence of Pyridine but approximately 100% Walden inversion takes place in the presence of pyridine.
(iii) Order of reactivity of alcohols is allyl ≅ benzyl > 3° > 2° > 1° > CH3OH
(iv) 1° alcohols and CH3OH react with HX through SN2 mechanism.
(v) 3°, 2° and hindered 1° alcohols follow SN1 mechanism.
(e) Reaction of HCl/ZnCl2 (Lucas reagent) called as Groove reaction gives immediate white turbidity with 3° alcohols, it takes 2 to 10 minutes for 2° alcohols while 1° alcohols react only on heating. This reaction is also known as Lucas test. 3° alcohols follow SN1 mechanism even in the absence of ZnCl2.
(f) With HI (or) P(red)/I2, when ethylene glycol reacts, the product is ethene excess of HI may convert it to ethyl iodide.
C. Dehydration:
(i) Ease of dehydration of alcohols is in the order : 3° > 2° > 1°, conjugation makes the reaction still easier. Saytzeff rule is followed. When dehydration is in one molecule, it is E1 elimination. 3°–alcohols are also dehydrated easily because 3° carbocation is formed as intermediate.
(a) 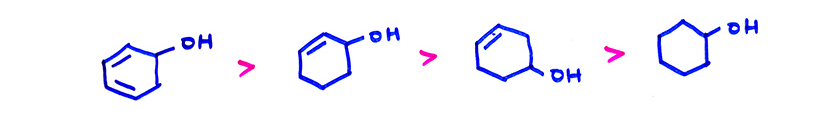
(b) 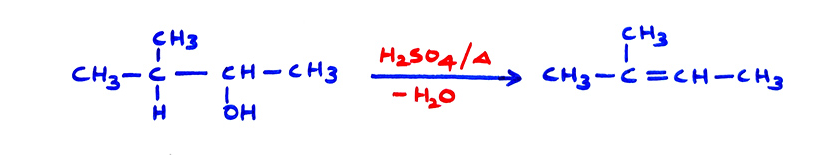
(c) \tt CH_3CH_2-OH \xrightarrow[(or)H_2SO_4/333-353K]{Al_2O_3/575-623K}C_2H_4+H_2O
D. Iodoform Test (Preparation of haloforms):
The reaction is given by compounds containing 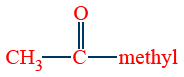 ketonic (or)
ketonic (or) 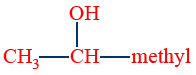 methyl carbinol group
methyl carbinol group
(a) \tt CH_3CH_2-OH+4I_2+6NaOH\xrightarrow{\Delta}CHI_3(Iodoform\ yellow\ solid)+HCOONa+5H_2O+5NaI
E. Acetal formation:
Monohydric alcohols react with aldehydes easily to produce hemiacetals and then acetals in the presence of HCl gas. Ketones give poor yield of hemiketals and ketals. Polyhydric alcohols react with aldehydes and ketones both to produce cyclic acetals and cyclic ketals repectively.
(a) 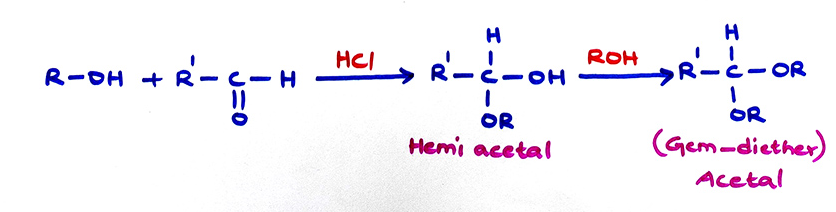
(c) If NaOH is used, only hemiacetal is formed.
(d) Acetals and hemiacetals can be hydrolysed by dil HCl
(e) Cyclic structures of monosaccharides are hemiacetals while those of disaccharides are acetals.
G. Oxidation:
(i) Oxidation of monohydric alcohols:
(a) \tt R-CH_2-OH(1^0alcohol)\xrightarrow{Cu/573K}R-CHO(Aldehyde)+H_2(Dehydration)
(b) \tt CH_3OH+\frac{1}{2}O_2\xrightarrow{Cu/823K}HCHO+H_2O
(c) \tt CH_3-CH_2-OH+O_2\xrightarrow[bacteria]{Mycodermaceti}CH_3COOH+H_2O(Quick\ vinegar\ process)
Trick for oxidation (List of reagents)
(f) Following chemicals oxidise 1° alcohols to acids, 2° alcohols to ketones
(i) 1% alkaline kMnO4 (Bayer's reagent)
(ii) K2Cr2O7/H2SO4 (dil), i.e, H2CrO4 (hypothetical acid)
(iii) KMnO4/H2SO4 (dil)
(iv) CrO3 / glacial CH3COOH
All these reagents can oxidise ketones under vigorous conditions to smaller acids.
(g) Following chemicals oxidise 1° alcohols to corresponding aldehydes (not to acids) and 2° alcohols to ketones
(i) CrO3/H2SO4 dil/Aq.acetone (Jone's reagent)
(ii) PCC : Pyridinium chlorochromate (C5H5NH+) \tt (ClCrO_3^{^-}) in CH2Cl2 (Sarett-collin reagent)
(iii) Pyridine + HCl + CrO3 (Corey's reagent)
(iv) PDC: Pyridinium dichromate (C5H5N+H)2 \tt (Cr_2O_7^{2-})
Pyridine + K2 Cr2O7 + H2SO4 dil
\tt R-CH_2OH\xrightarrow[\Delta]{(i)or(ii)or(iii)}R-CHO
NBS also oxidises 1° alcohols to aldehydes and 2° alcohols to ketones.
(h) Tertiary butoxide of aluminium [(CH3)3CO]3 Al in acetone (or) cyclohexanone converts 1° alcohols to aldehydes and 2° alcohols to ketones at the cost of acetone or cyclohexanone that is reduced to propan-2-ol or cyclohexanol. The reaction is known as oppenauer oxidation.
(i) MnO2 is used to oxidise allylic and benzylic alcohols to corresponding aldehydes and ketones.
(a) \tt CH_2=CH-CH_2OH\xrightarrow{MnO_2/\Delta}CH_2=CH-CHO
(j) N2O4/CHCl3, Field's reagent converts only benzylic alcohols to corresponding aldehydes and ketones
Oxidation of Phenol:
(a) 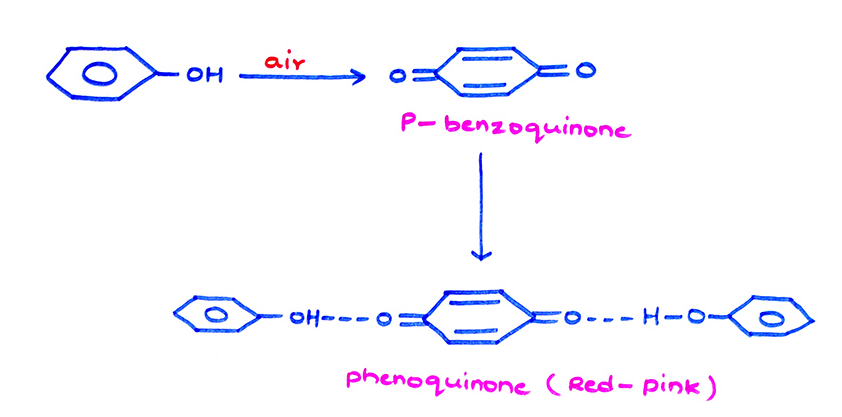
Properties of Phenol due to benzene ring: 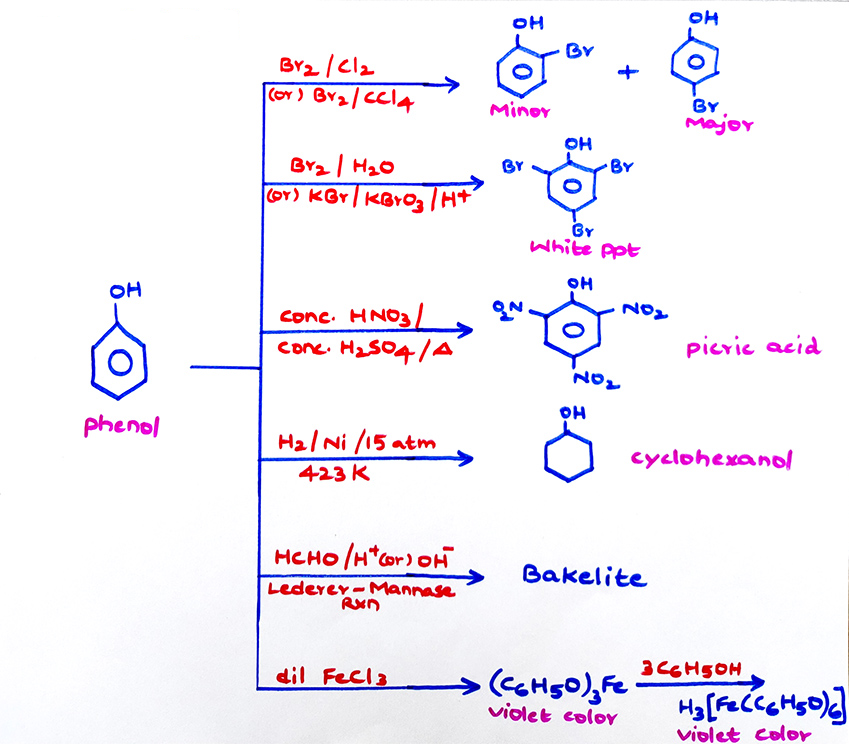
Distinctions among 1°, 2° and 3° alcohols:
Victor Meyer's test (Red-blue test)
| Reagent Used | 1° Alcohol R–CH2–OH |
2° alcohol R2CH–OH |
3° alcohol R3 C–OH |
| P/I2 | R–CH2–I | R2CHI | R3C–I |
| AgNO2 | RCH2NO2 | 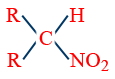 |
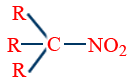 |
| HNO2 (NaNO2/HCl) |
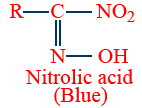 |
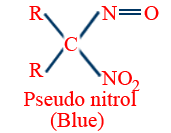 |
No reaction |
| NaOH | 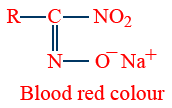 |
No reaction Blue colour retained |
No reaction No colour |
Solubility of Alcohols:
(a) Solubility of alcohols increases with increase in branching and degree of alcohol.
n-butyl alcohol < isobutyl alcohol < sec-butylalcohol < ter-butyl alcohol
(b) Boiling points of alcohols decrease with increase in degree of alcohol and increase with increase in length of the chain (hydrocarbon part)
methanol < ethanol < propan-2-ol < propan-1-ol
Part1: View the Topic in this Video from 0:08 to 14:26
Part2: View the Topic in this Video from 0:08 to 27:44
Part3: View the Topic in this Video from 0:08 to 24:04
Part4: View the Topic in this Video from 0:08 to 5:53
Part5: View the Topic in this Video from 0:07 to 13:09
Part6: View the Topic in this Video from 0:08 to 13:55
Part7: View the Topic in this Video from 0:09 to 11:55
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

