Chemical Bonding and Molecular Structure
VSEPR Theory and Geometry of Molecules
VSEPR Theory :
The valency shell is assumed as a sphere and the electron pairs are arranged around the central atom.
A multiple bond is treated as single super electron pair.
Repulsion lp – lp > lp – bp > bp – bp
ex ; sp2 type → No. of Bp's → 2, Lp's → 1
Geometry → Trigonal planar
Shape → Angular
Ex : SnCl2, HgCl2
| Hybridization | No. of Bp's | No. of Lp's | Geometry | Shape | Example |
| sp3 | 4 | - | Tetrahedron | Tetrahedron | CH_{4}, CCl_{4},SO_4^{-2},NH_4^+ |
| sp3 | 3 | 1 | Tetrahedron | Pyramid | NH_{3}, NCl_{3},PH_{3},SO_3^{-2} |
| sp3 | 2 | 2 | Tetrahedron | V- Shape, bent shape | H_{2}O, OF_{2},Cl_{2}O |
| Hybridization | No. Bp's | No. of Lp's | Geometry | Shape | Example |
| sp3d | 5 | 0 | Trigonal bipyramidal | Trigonal bipyramidal | PF_{5}, PCl_{5} |
| sp3d | 4 | 1 | Trigonal bipyramidal | see saw | SF_{4}, SCl_{4} |
| sp3d | 3 | 2 | Trigonal bipyramidal | T - shape | BrF_{3}, RCl_{3},ClF_{3} |
| sp3d | 2 | 3 | Trigonal bipyramidal | Linear | I_3^-,XeF_{2},ICl_2^{-} |
| sp3d3 | 7 | 0 | Pentagonal bipyramidal | Pentagonal bipyramidal | IF_{7} |
| sp3d3 | 6 | 1 | Pentagonal bipyramidall | Distorted octahedron | XeF_{6} |
Short cut hybridization formula :
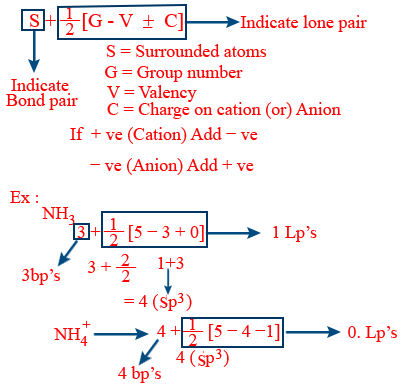
Formal charge :
F.C = valence electrons − (no. of lone pair electrons + no. of bond pairs)
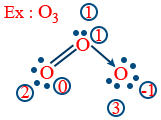
1) Oxygen atom
6 − (2 + 3)
6 − 5 = 1
2) 2nd oxygen
6 − (4 + 2) = 0
3) 3rd oxygen
6 − (6 + 1) = 6 − 7 = −1
Part1: View the Topic in this Video from 0:49 to 56:39
Part2: View the Topic in this Video from 0:40 to 4:52
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

