Haloalkanes and Haloarenes
Physical and Chemical Properties of Haloalkanes
- Physical properties of Haloalkanes:
(a) Insoluble in water, though polar, because of being incapable of forming H-bond.
(b) Branches decrease boiling points. 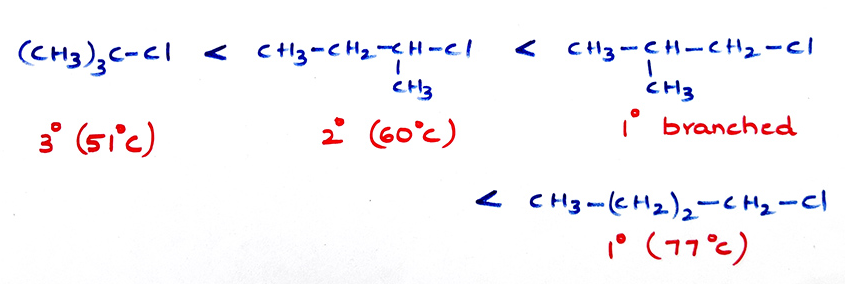
- (c) Boiling point for same halogen increase in R group CH3Cl < CH3CH2Cl < CH3CH2CH2-Cl
(d) Boiling point increase for same alkyl group as I > Br > Cl > F
C2H5I > C2H5Br > C2H5Cl > C2H5F
(e) Density increases with increase in number of halogens and mass of halogen
C2H5I > C2H5Br > C2H5Cl > C2H5F ; CH3Cl > CH2Cl2 > CHCl3 > CCl4
(f) Dipole moment
CH3Cl (1.860D) > CH3F (1.847 D) > CH3Br (1.830D) > CH3I (1.636 D)
(g) Stability: RF > RBr > RCl > RI
(h) Alkyl iodides become dark due to the formation of I2
2RI → R − R + I2 - Some physical properties of haloarenes:
(a) Insoluble in water due to lack of formation of H-bond.
(b) Density and b.p. same as in case of haloalkanes.
(c) Dipole moment: 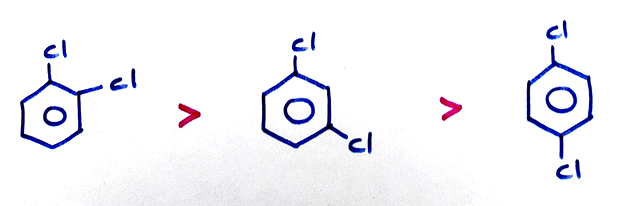
(d) O-Dichloro benzene has less dipole moment than the expected value because the steric effect of two Cl-atoms at -O- positions increases the bond angle than 60°.
(e) P-Dichloro benzene is used as antimoth compound and room freshener in ODONIL.
(f) Melting points: P-Dichloro benzene, because of best packing in solid state, among dichloro benzenes, has highest m.p 53°C and meta isomer the lowest 33°C, O-Dichloro benzene melts at 43°C
(g) Boiling points: O-Dichloro benzene (179°C) > para isomer (172°C) ≈ meta isomer.
View the Topic in this Video from 30:30 to 36:40
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

