d - and f -Block Elements
Important Compounds of Transition Elements
- Preparation of K2Cr2O7:

- Na2CrO4 is extracted with water as yellow solution and acidified by H2SO4 till pH is close to 4
(ii) 2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
After removal of Na2SO4 · 10H2O, the solution is boiled with KCl.
(iii) Na2Cr2O7 + 2 KCl → 2 NaCl + K2Cr2O7
Orange coloured crystals of K2Cr2O7 (m.p. 398° C) are collected.
(iv) Na2Cr2O7 is hygroscopic and is therefore, converted to stable K2Cr2O7. - Properties of K2Cr2O7:
(a) Effect of heat : 4K2Cr2O7 → 4 K2CrO4 + 2Cr2O3 + 3O2
(b) Change of pH : 
- (c) Action of H2SO4:
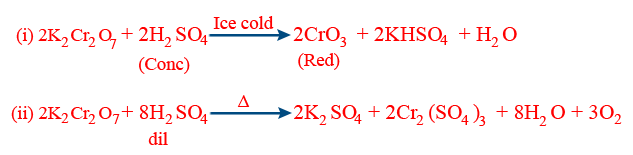
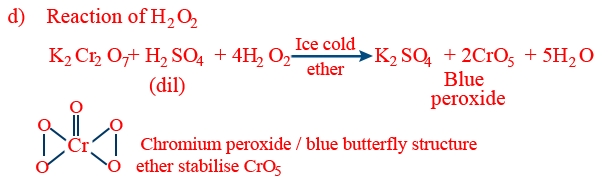
- (f) Chromyl chloride test:
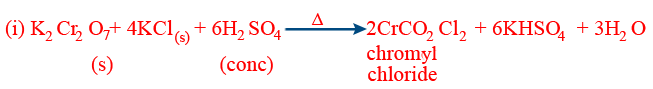
(ii) Orange-red fumes of chromyl chloride are dissolved in NaOH to obtain Na2CrO4, yellow solution which gives yellow precipitates of PbCrO4 an reacting with (CH3COO)2 Pb
(iii) CrO2Cl2 + 2NaOH → Na2CrO4 +2HCl
Na2CrO4 + (CH3COO)2 Pb → PbCrO4 +2 CH3COONa
(iv) This test is not given by HgCl2 and other covalent chlorides.- (g) Oxidizing properties: Solution turns green due to the formation of Cr3+ [Cr2(SO4)3]
\tt Cr_{2}O_7^{2-}+14 H^{+}+6e^{-}\rightarrow 2Cr^{3+}+7H_{2}O
\tt Eq.wt = \frac{Mol.wt\ of\ M}{6}
(i) Molecular equation K2Cr2O7 + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 4 H2O + 3[O]
(ii) Oxidation of HCl : \tt Cr_{2}O_7^{2-}+8H^{+}+6HCl \rightarrow 2Cr^{3+}+7H_{2}O+3Cl_2
(iii) Oxidation of I−: \tt Cr_{2}O_7^{2-}+14H^{+}+6I^{-} \rightarrow 2Cr^{3+}+7H_{2}O+3I_2
(iv) Oxidation of Fe2+: \tt Cr_{2}O_7^{2-}+14H^{+}+3H_2S \rightarrow 2Cr^{3+}+7H_{2}O+3S
(v) \tt SO_3^{2-} \ to \ SO_4^{2-}:Cr_{2}O_7^{2-}+8H^{+}+3SO_3^{2-} \rightarrow 2Cr^{3+}+3SO_4^{2-}+4H_{2}O
(vi) SO2 to H2SO4: \tt Cr_{2}O_7^{2-}+3SO_{2}+2H^{+} \rightarrow 2Cr^{3+}+H_{2}O+3SO_4^{2-}
(vii) Chrome alum [K2SO4. Cr2(SO4)3. 24H2O] used for tanning of leather is collected from this solution by crystallization.
(viii) Sn2+ to Sn4+ : \tt Cr_{2}O_7^{2-}+3Sn^{2+}+14H^{+} \rightarrow 2Cr^{3+}+3Sn^{4+}+7H_{2}O
(ix) Oxidation of ethanol: \tt C_{2}H_{5}OH\xrightarrow[-H_{2}O]{{[O]}}CH_{3}CHO\xrightarrow[]{{[O]}}CH_{3}COOH. - Preparation of KMnO4:
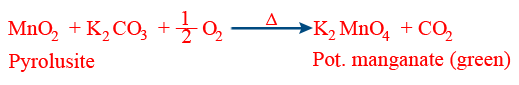
\tt MnO_{2}+2KOH+\frac{1}{2}O_{2}\rightarrow K_{2}MnO_{4}+H_{2}O
(b) 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl
2K2MnO4 + H2O + O3→ 2KMnO4 + 2KOH + O2
3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3
(c) Electrolytic oxidation : \tt MnO_4^{2-}\rightarrow MnO_4^{^-}+e^{-}- Properties of KMnO4 :
(a) Effect of heat: \tt 2KMnO_4 \xrightarrow[]{{473^{o}C}} K_{2}MnO_4+MnO_{2}+O_{2} 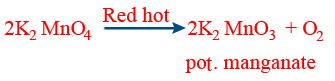
(b) Action of H2SO4:
(ii) If KMnO4 is in excess (MnO3)2SO4 changes to oily explosive Mn2O7
(MnO3)2 SO4 + H2O → Mn2O7 + H2SO4:
(iii) The net reaction in case of excess of KMnO4 solid is 2KMnO4 + 2H2SO4 → 2KHSO4 + Mn2O7 + H2O
(iv) Mn2O7 explodes if the solution is not ice cold Mn_{2}O_{7}\rightarrow 2MnO_{2}+\frac{3}{2}O_{2}
(c) Oxidising properties:
(i) Acidic medium:
(a) \tt MnO_4^\ominus + 8H^{+}+5e^{-}\rightarrow Mn^{2+}+4H_{2}O
Equivalent mass = \tt \frac{Mol\ wt \ of \ M}{5}
(b) Molecular equation 2 KMnO4 + 3H2SO4 (dil) → K2SO4 + 2MnSO4 + 3H2O + 5[O]
(c) In acidic medium KMnO4
Oxides I− to I2
Fe2+ to Fe3+
H2S to S; \tt SO_3^{2-} \ to \ SO_4^{2-}
SO2 to H2SO4
Sn2+ to Sn4+ :
\tt NO_2^{^-} \ to \ NO_3^{^-}
HCl to Cl2
alcohols to acids as K2Cr2O7 / H+ does.
(d) \tt 2MnO_4^{^-} + 6H^{+}+10 \ HCl \rightarrow 2Mn^{2+}+8H_{2}O+5Cl_{2}
(e) In Lab KMnO4 / H+ is specifically used to estimate the strength of Mohr's salt (NH4)2 SO4 · FeSO4 · 6H2O and oxalic acid.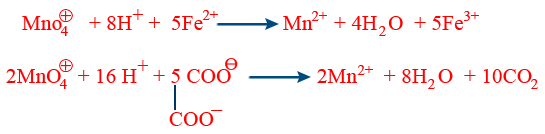
(f) Oxidation of FeC2O4 : Here, both the ions are oxidised. The same is the case of Fe(NO2)2
\tt 3MnO_4^{^-} + 24H^{+}+5FeC_{2}O_{4} \rightarrow 3Mn^{2+}+12H_{2}O+5Fe^{3+}+10CO_{2}
(ii) Neutral medium:
\tt MnO_4^{^-} + 2H_{2}O+3e^{-}\rightarrow MnO_{2}+4OH^{^-}
Equivalent mass = \tt \frac{Mol \ wt \ of \ M}{3}=\frac{M}{3}
(b) Molecular equation 2 KMnO4 + H2O → 2KOH + 2MnO2 + 3[O]
(c) Oxidation of H2S
2KMnO4 + 3H2S → 2KOH + 2MnO2 + 3H2O + 3S
(d) Oxidation of Na2S2O3
2KMnO4 + 3Na2S2O3 + H2O → 2KOH + 2MnO2 + 3Na2SO4 + 3S
(e) NH3 to N2
2NH3 + 2 KMnO4 → 2KOH + 2MnO2 + 2H2O + N2
(iii) Alkaline medium (1% alk.KMnO4 is called Baeyer's reagent.)
(a) Purple solution (Mn7+) changes to green (Mn+6) and then to brown due to the formation of Mn4+ as MnO2
\tt MnO_4^{^-}+1e^-\rightarrow MnO_2^{2-}
Equivalent mass = \tt \frac{Mol \ wt \ of \ M}{1}=M.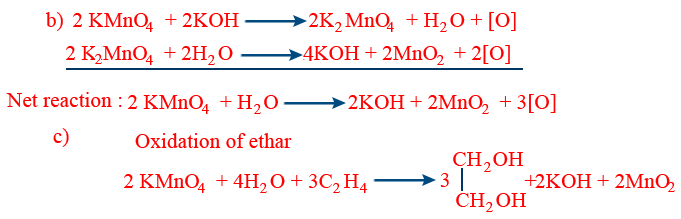
- (f) Oxidation of KI
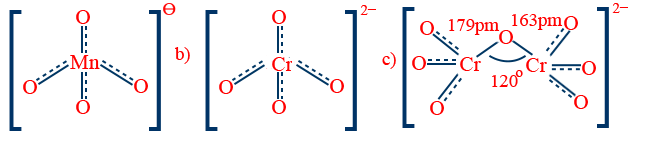
2 KMnO4 + H2O + KI → 2KOH + KIO3 + 2MnO2 - Structures of \tt MnO_4^{^-},CrO_4^{2-} and \tt Cr_{2}O_7^{2-} : In both central atom undergoes d3s hybridization {i.e. 3dxy 3dyz 3dzx 4s}
View the Topic in this Video from 0:10 to 17:37
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

