Co-ordination Compounds
Ligands, Co-ordination number, Denticity and Chelation
Co ordination number:
Total number of electron pairs donated by the central metal atom through coordinated bond. Depending on the valencies of atoms coordination no. is defined.
ex:
| ion | CN |
| Cr+1 | 6 |
| Sn+4 | 6 |
| Pb+4 | 6 |
| Pt+4 | 6 |
Sidgwick Rule of Effective Atomic Number:
E.A.N = Atomic number − oxidation number + 2 coordination number
(or)
E.A.N = No. of electrons on central metal + electrons donated by ligands / metal / bridge ligand
Z = Atomic number, o.s → oxidation state
C.N = Coordination number of central metal.
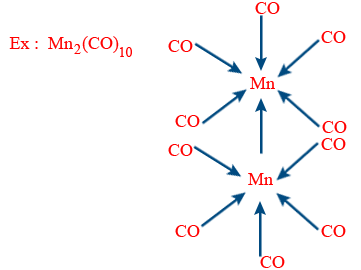
E.A.N (Mn) = 25 − 0 + 2 × 5 + 1 = 36
Part1: View the Topic in this Video from 19:27 to 59:16
Part2: View the Topic in this Video from 0:40 to 12:25
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. In case of monodentate ligands,
Coordination number = number of ligands
2. In polydentate ligands,
Coordination number = number of ligands × denticity
3. Effective Atomic Number (EAN)
This concept was proposed by Sidgwick. In a complex, the EAN of metal atom is equal to the total number of electrons present in it.
EAN = Z − ON of metal + 2 × CN
(where, Z = atomic number of metal atom
ON = oxidation number of metal
and CN = coordination number of complex)

