Co-ordination Compounds
Bonding, Valence Bond Theory and Crystal Field Theory, Colour, Magnetic Properties and Shapes
Valence Bond Theory:
1. According to VBT, ligands form coordination bond with vacant orbitals of the central metal atom or ion.
2. Type of hybridisation, bond angle and magnetic moment of the complex compound on the nature of the ligand.
3. Ligands are arranged in the order of their increasing CFSE
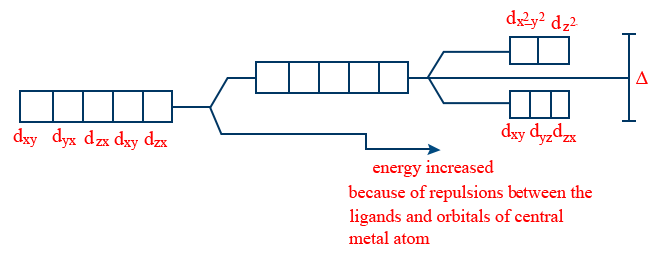
Note:
Weak field ligands: The ligands which affect only a small degree of crystal field splitting.
I− < Br− < SCN− < S−2 < NO3− < F− < OH− < EtOH < CH3COO− \tt \approx C_2O_4^{-2} < H2O < EDTA
Strong field ligands:
They affect a large degree of crystal field splitting.
NCS− < NH3 ≈ py <en < diphy < NO2− < CN− < CO
NO2− , CN− and CO have synergy bonding (or) Back bonding.
Strength of donor sites:
X− < O− < N− < C−
P = Pairing energy of electrons
Δo < P, then the ligands is a weak field ligand or high spin ligand.
Δo > P, then the ligand is a strong field ligand or low spin ligand.
Postulates of VBT
1. Complexes which have no unpaired electron - diamagnetic
2. Complexes which have unpaired electron - Paramagnetic
3. Spin only magnetic moment = \tt \sqrt{n\left(n+2\right)} B.M, n → unpaired electrons.
| Type of hybridisation | Shape | Geometry | Bond angle |
| sp | Linear |  |
180° |
| sp2 | Trigonal planar | 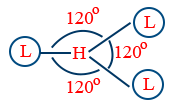 |
120° |
| dsp2 (inner) |
Square planar | 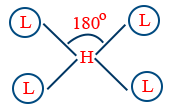 |
|
| sp3 (outer orbital) |
Tetrahedral | 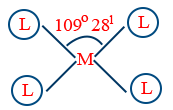 |
109°28' |
| dsp3 (n−1)d ns np sp3dx2−y2 ns np nd dsp3 x2 − y2 |
Square pyramidal |  |
90° |
| sp3d2 (outer orbital complex) d2sp3 (inner orbital complex) |
Octahedral |  |
90° |
If there is only one electron in the last d - orbital (co-ordination no = 4) it can shift the electron and form dsp2 (square planar)
If there is only one electron in the last but one d-orbital (coordination = 6) and no of e− in the last orbital, shift the e− and form d2sp3 (octahedral)
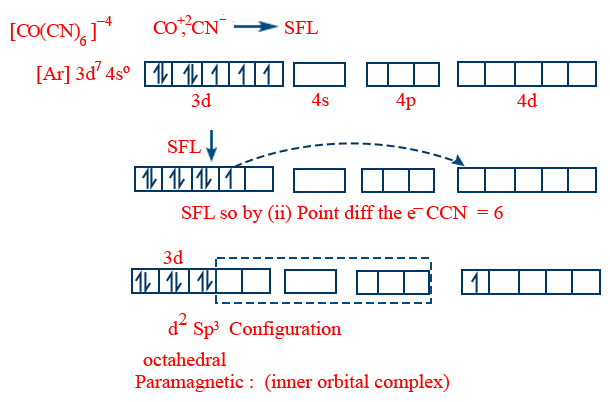
Crystal Field Theory:
Complex formation is due to combination of a central metal ion surrounding by ligands which act as point charges or point dipoles. Arrangement is such that repulsion between central metal 'M' and ligand is minimum.
Interaction force between the positively charged nucleus of M and negatively charged ligands is of 2 types.
a. Attractive force: between 'M' cation and negatively charged ligand / −ve end of polar neutral ligand.
b. Repulsive force: between the lone pair of ligand and electrons of d-orbitals of 'M'.
Thus force is responsible for the relative energies of the d-orbitals of the central metal ion or atom.
Octahedral Complex: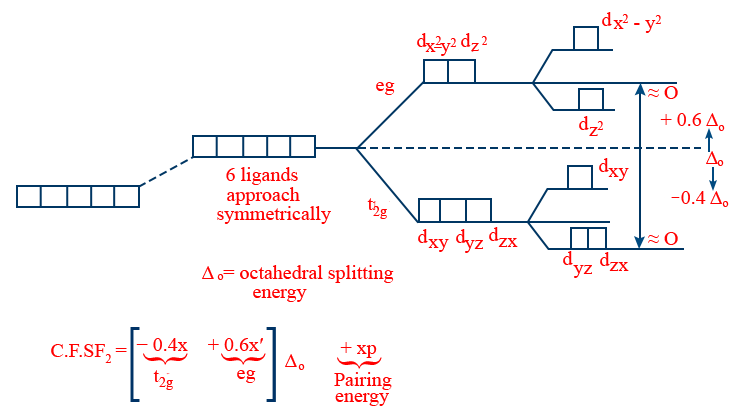
x,x' → no. of electrons in t2g, eg respectively.
Octahedral complex: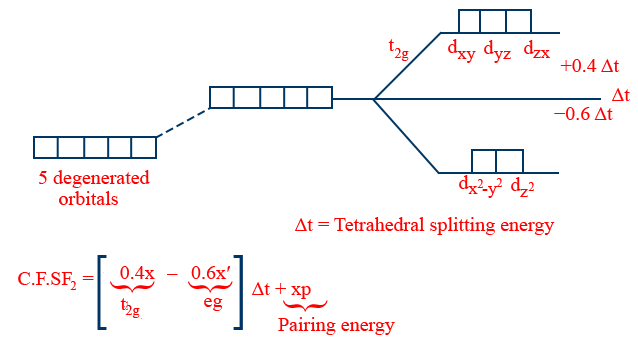
Note: for same ligand and central metal atom/ion
i. \tt \Delta t= \frac{4}{9}\ \Delta_0
ii. Δsp = 1.3Δ0
Colour of complexes:
Splitting energy per mole = \tt N_A\left(\frac{hc}{\lambda}\right)kJ/mole
λ → tells us colour absorbed.
One side → colour absorbed
other side → colour emitted
If λ lies outside the visible region, transmitted light is colourless. Hence complex is also colourless.
Low spin / high spin complexes:
If configuration does not change along with the nature of ligand, then we can't decide low spin / high spin
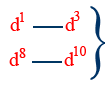 Configuration does not change for WFL or SFL in case of octahedral complex
Configuration does not change for WFL or SFL in case of octahedral complex
d4 — d7 → WFL (high spin complex)
→ SFL (low spin complex)
| Ligand | IUPAC Name | Co-ordination sites |
| CN− | cyanido | Monodentate (Ambidentate ligand) |
| NC− | Isocyanido | Monodentate (Ambidentate ligand) |
| N3− | nitrido | Monodentate |
| \tt N_3^{^-} | azido | Monodentate |
| \tt NH_2^{^-} | amido | Monodentate |
| NH2− | imido | Monodentate |
| SCN− | thiocyanato | Monodentate (Ambidentate ligand) |
| NCS− | Isothiocyanato | Monodentate (Ambidentate ligand) |
| NO+ | nitro oxium | Monodentate |
| \tt C_2O_4^{-2} | oxalato | Bidentate |
Part1: View the Topic in this Video from 0:40 to 59:35
Part2: View the Topic in this Video from 0:40 to 1:01:58
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

