Structure of Atom
Nature of electromagnetic radiation and Photoelectric effect
Nature of Light : Different Theories
Newton's Corpuscular Theory :
Light comes out of the source as small particles called corpuscles. It traces in straight lines.
Huygen's Wave Theory :
According to Huygen, light has wave character. He compared light with mechanical waves.
Maxwell's Electromagnetic Theory :
A reaction which consists of both electric and magnetic components which are perpendicular to each other and perpendicular to direction of propagation of light

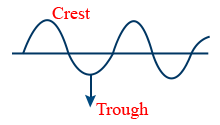
Wave length (λ) : Units cm/s ; m/sec
Frequency(ν) : \tt \nu = \frac{C}{\lambda}
units Hz (or) Cps (or) S-1
eg : λ400nm = 400 × 10-9 = 4 × 10-7 m
wave number ( \overline{\nu}) :
\overline{\nu} = \frac{1}{\lambda}
units = cm-1 (or) m-1
Velocity of light (c) :
C = 3 × 108 m/s (or) 3 × 1010 cm/sec
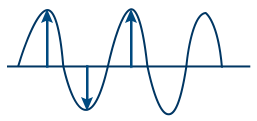
Amplitude (A) : I ∝ a2
I = Intensity
Electromagnetic spectrum :
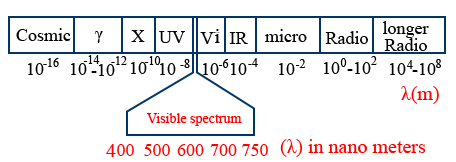
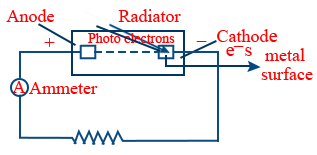
Photo electric effect : The emission of electrons by a metal surface when a radiation having same frequency
h\nu = W + KE \ \ \ \ \ \ \ \ KE = \frac{1}{2}mv^{2}
W → Work function (The amount of energy required to free the electron from the metal surface)
W = hν0 → The minimum frequency
ν0 = Threshold frequency → The minimum frequency required to free the electron.
Part1: View the Topic in this Video from 33:09 to 56:12
Part2: View the Topic in this video From 0:40 to 43:40
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. Frequency = \tt V=\frac{C}{\lambda}
2. Wavelength = \tt \lambda=\frac{C}{V}
3. Wave number = \tt \overline{v}=\frac{1}{\lambda}, v=\frac{c}{\lambda}=c\overline{v}
4. Time period \tt T=\frac{1}{\upsilon}
5. No. of waves \tt n=\frac{2\pi r}{\lambda}\left(where \ \lambda=\frac{h}{m\upsilon}\right)
6. No. of revolutions of e- per second is =\tt \frac{\upsilon}{2\pi r}

