Hydrocarbons
Aromatic Hydrocarbons and Carcinogenicity
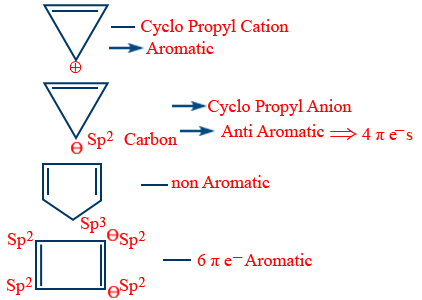
Aromatic compounds :
They should obey the following Huckel's rule. i.e., (4n + 2)π electron. Here n = 0, 1, 2, 3 -------. It is also called (4n + 2) rule.
ex : Benzene C6H6 - 6π electrons.
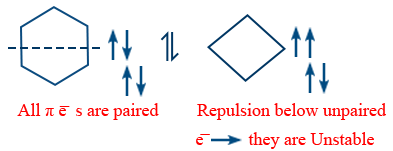
Anti aromatic compounds :
1. Molecule is cyclic, planar it should contain 4π e−
In the ring at each atom 'P' - orbital should be present.
ex : Cyclo butadiene
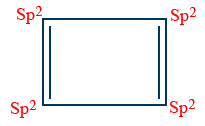
Non - aromatic compounds :
All atoms should not contain P - orbitals in the ring.
Stability order
Aromatic > Non aromatic > Anti aromatic
Stability between aromatic and anti aromatic
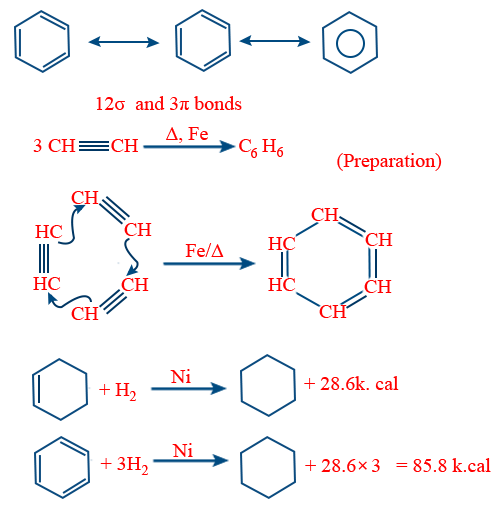
Nitration :
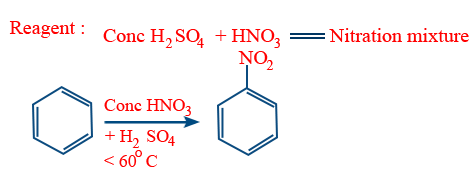
For nitration reactivity order
C6H6 = C6D6 = C6T6
N2O5 solid \left(NO_2^+ , NO_3^{-}\right)
Fuming nitric acid (fumes of NO2 with conc HNO3)
NO_2^+ , ClO_4^{-}
AC_{2}O + N_{2}O_{5}
↓
(CH_{3}CO)_{2}
Sulphonation :
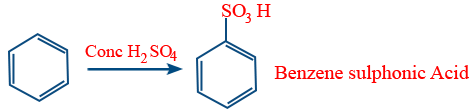
Rate of sulphonation :
C6H6 > C6D6 > C6T6
Ortho para directing (or) Activating groups :
OH, NH2, NH3, NHCOCH3, OCH3, CH3, C2H5 ----- etc
Meta directing groups / Deactivating groups :
NO2, CN, CHO, COR, COOH, COOR, SO3H ----- etc.
Note : (Halogens)Cl ⇒ Deactivating group ⇒ inductive effect ⇒ O and P directing group ⇒ resonance.
Part1: View the Topic in this Video from 0:07 to 7:51
Part2: View the Topic in this Video from 0:07 to 3:51
Part3: View the Topic in this Video from 0:07 to 14:27
Part4: View the Topic in this Video from 0:07 to 17:30
Part5: View the Topic in this Video from 0:07 to 16:50
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

