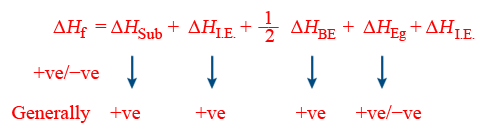Chemical Bonding and Molecular Structure
Calculation of lattice enthalpy and Concept of electronegativity
Lattice Energy :
The amount of energy released during the formation of 1mole of ionic crystal from its constituent gaseous ions is called lattice energy
u \propto \frac{q_{1}q_{2}}{r^{+} + r^{-}}
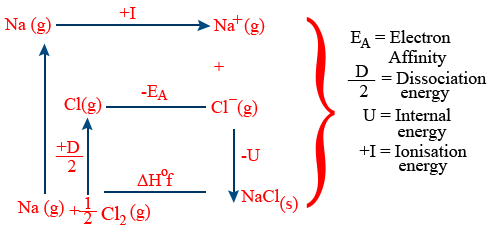
Born Haber cycle(Calculation of lattice energy)
Hydration energy :
The process in which cation and anion are surrounded by H2O molecules is called hydration energy.
If HE > LE soluble
HE < LE insoluble
Part1: View the Topic in this Video from 0:12 to 13:54
Part2: View the Topic in this Video from 0:11 to 10:56
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.