Surface Chemistry
Classification and Preparation of Colloids, Suspensions
- Colloidal state (THOMAS GRAHAM):
- Colloids: Solute particles when present in solution and cannot pass through animal membrane (or) parchment paper are called colloids.
- Colloidal solutions are actually heterogeneous mixtures with some dispersion medium, in which the size of dispersed phase (solute) particles is between 1 to 1000 nm. i.e., 10−9 to 10−6 m.
- Comparison with crystalloids (true solutions) and suspensions:
| S.No. | Property | True solution | Colloidal solution | Suspension |
| 1. | Size of particle | < 1 nm | 1 to 1000 nm | > 1000 nm |
| 2. | Nature | Homogeneous | Heterogeneous | Heterogeneous |
| 3. | Visibility of particles | Particles not visible | Visible under ultra microscope | Visible to naked eyes |
| 4. | Appearance | Clear and transparent | Translucent | Opaque |
| 5. | Setting of particles | Do not set under gravity | Extremely poor setting under gravity, but set by ultra centrifugation. |
Settle under gravity. |
| 6. | Filtration | Pass through filter paper as well as animal membrane and parchment paper | Do not pass through parchment paper or animal membrane but pass through filter paper. | These are retained by filter paper as well as parchment paper or animal membrane |
| 7. | Examples | Solutions of sugar salt etc. in water | Milk, blood, starch solution etc. | Muddy water precipitates in reactions etc. |
- Classification of colloids: Based on physical state of dispersed phase and dispersion medium colloids are of eight kinds.
| S.No. | Dispersed phase (D.P) | Dispersion medium(D.M) | Type | Examples |
| 1. | Solid | Solid | Solid sol | Gem stones, coloured glasses. |
| 2. | Solid | Liquid | Sol | Paints, blood. |
| 3. | Solid | Gas | Aerosol of solid | Smoke, dust storm |
| 4. | Liquid | Solid | Gel | Butter, jellies, cheese |
| 5. | Liquid | Liquid | Emulsion | Hair & face creams, milk |
| 6. | Liquid | Gas | Aerosol of liquid | Insecticide spray, clouds, fog |
| 7. | Gas | Solid | Solid sol | Foam rubber, pumice stone. |
| 8. | Gas | Liquid | Foam | Soap suds, whipped cream. |
- (*) Gas in gas colloids are not possible as gaseous mixtures are true solutions.
- Classification of sols based on interaction between D.P and D.M:
(a) Solvent attracting sols are called lyophilic sols.
(b) Solvent repelling sols are called lyophobic sols.
| S.No. | Property | Lyophilic colloids (emulsoids) | Lyophobic colloids (suspensoids) |
| 1. | Affinity for medium | Very high | Quite low (or) nil |
| 2. | Formation | Direct mixing of D.P and D.M | Special methods are required. |
| 3. | Stability | Highly self stable | Much less stable and require stabilisers for stability. Easily coagulated. |
| 4. | Reversibility | Reversible | Irreversible |
| 5. | Viscosity | Higher than D.M | Equal to that of D.M |
| 6. | Surface tension | Lower than D.M | Equal to that of D.M |
| 7. | Electrophoresis | Not shown generally | Shown due to charge on particles |
| 8. | Examples | Starch, gelatin, protein sols | Sols of metal As2, S3, S8, Fe(OH)3 etc. |
- Classification of sols based on medium used:
| Medium | Name given to sol |
| Water
Alcohol Benzene Air |
Aquasol
Alcosol Benzosol Aerosol |
- Classification of colloids based on size of D.P and colloidal particles:
(a) Macro molecular colloids: The size of D.P. particles is quite large and have high molecular masses. Eg: Starch, gelatin, enzymes, rubber sols etc.
(b) Multi molecular colloids: The size of D.P. particles is less than 1 nm which aggregate to give colloidal particles. Eg: gold, sulphur sols etc.
(c) Associated colloids (or) micelles:
(i) Some substances act as strong electrolytes but at higher concentrations change into colloids. These colloids are called associated colloids or micelles.
(ii) Micelles are formed above certain minimum concentration called critical micelle concentration (CMC) & above a specific temperature called kraft temperature (TK).
(iii) CMC for soap is 10−4 to 10−3 mole L−1 . A micelle of soap contains 100 (or) more RCOO− ions. At CMC the non-polar chains i.e., hydrophobic tails are pulled inside and polar ends COO− outside to give ionic micelle. 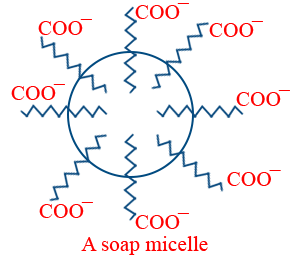
- Preparation of Lyophilic sols:
(i) Lyophilic colloids are very stable and can be prepared by shaking the D.P. in D.M. Eg: gelatin, gum, starch etc. under hot or cold conditions
(ii) Sol of cellulose nitrate in ethanol is called collodion. - Preparation of lyophobic sols:
- (1) Condensation methods: These methods involve chemical reaction, exchange of solvent, excessive cooling etc.
(i) Br2(aq) + H2S(g) → 2HBr + S(sol) oxidation of S2− to S.
(ii) 2 AuCl3(aq) + 3 SnCl2(aq) → 2 Au(sol) + 3SnCl4 Reduction of Au3+ to Au.
(iii) FeCl3(aq paste) + 3H2O(boiling) → Fe(OH)3(sol) + 3HCl Hydrolysis of FeCl3
(iv) As2O3(aq) + 3H2S(g) → As2S3(sol) + 3H2O Double decomposition.
(v) P (or) (Solution in alcohol) + H2O(excess) → Sol of P (or) P' Exchange of solvent - (2) Disintegration (or) dispersion methods:
(a) Bredig's method (or) electric disintegration: An electric arc is stuck between two electrodes of a metal, whose sol is to be prepared, after immersing in the suitable ice cooled medium containing a suitable stabilizer for the sol. The heat produced vaporises (disintegrates) the metal which on condensation give colloid.
(b) Peptisation: (i) A freshly prepared precipitate is added in small quantities, with constant stirring, into a related electrolyte, to prepare colloidal solutions.
(ii) Red coloured Fe(OH)3 sol is easily prepared by peptisation of freshly prepared Fe(OH)3 precipitates in FeCl3(aq). Adsorption of Fe3+ ions on Fe(OH)3 precipitate breaks it to colloidal form.
(c) Mechanical disintegration: (i) Suspension of a D.P in a suitable D.M is passed through two concentric steel discs moving at about 7000 rotations per minute to prepare colloidal solution. The apparatus is commonly known as colloidal mill.
(ii) Printing ink, colloidal graphite etc are commonly made by this method. - Origin of charge on sol particles:
(a) Electron capture by sol particles during electro dispersion method.
(b) Frictional electrification.
(c) Preferential adsorption of ions.
(i) AgNO3 added to KI develops -ve charge due to adsorption of I− on the sol particles of AgI.
AgNO3 + 2KI(excess) → [AgI]I− + 2K+ + NO3− - (ii) Fe(OH)3 adsorbs Fe3+ to give +ve charged [Fe(OH)3]Fe3+
(d) Ionization of surface groups: Dyes ionise to give colloids
Acidic dye molecule → H+ + (colloidal particle)−
Basic dye molecule → OH− + (colloidal particle)+
(e) Colloids with +ve charged metal hydroxides eg: Cr(OH)3, Fe(OH)3, Al(OH)3, etc., oxides like TiO2 basic dyes, proteins in acidic medium etc.,
(f) Colloids with -ve charge: metals, eg, Cu, Ag, Au, Metal sulphides eg, As2S3, CdS etc., macro molecular colloids like starch, gum, etc., acid dyes eg, congo red etc., proteins in basic medium; albuminoids in blood. - Purification of sols:
(1) Ultra filtration: Ultra filters have pore size less than 1 nm. A colloid is collected is such filter papers and again dispersed in suitable medium.
(2) Ultra centrifugation: Colloidal particles are made to settle in a tube when colloidal solution is given ultra centrifugation. The particles are again dispersed in the suitable medium to prepare the sol again.
(3) Dialysis: It is the process of removing the ions of true solution from a colloidal solution by allowing them to pass through animal membrane or parchment paper when the colloidal particles are retained in it.
A bag of parchment paper containing the sol is placed in running distilled water in which ions from colloidal solution diffuse out.
(4) Electrodialysis: The process of removal of ions of true solution from a colloidal solution using parchment / cellophane paper can be enhanced by using electric potential. The process is called electrodialysis.
This method is used for the purification of blood by using artificial kidney machine.
Part1: View the Topic in this Video from 0:07 to 10:20
Part2: View the Topic in this Video from 0:09 to 16:15
Part3: View the Topic in this Video from 0:09 to 20:05
Part4: View the Topic in this Video from 0:09 to 13:20
Part5: View the Topic in this Video from 0:08 to 15:02
Part6: View the Topic in this Video from 0:08 to 23:10
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

