Solutions
Colligative Properties
Colligative properties :-
(1) The properties of dilute solution those depend on the number of solute particles irrespective to their nature.
(2) Colligative properties are classified into four types
a. Relative lowering of vapour pressure
b. Elevation of boiling point
c. Depression of freezing point
d. Osmotic pressure
(3) Normal colligative properties :-
When neither association nor dissociation of solute particle take place.
(i) Relative lowering of vapour pressure \tt \frac{P^{o} - P}{P} = X_{solute}
(ii) Elevation of boiling point ΔTb = kb m
(iii) Depression of freezing point ΔTf = kf m
(iv) Osmotic pressure π = CRT (or) π = CST
(i) Relative lowering of vapour pressure:-
\tt RLVP : \frac{P^{o} - P}{P^{o}} = X_{solute} = \frac{n}{n + N}
Trick:-
(a) For dilute solution (whose mass/mass % ≤ 5)
\tt \frac{P^{o} - P}{P^{o}} = \frac{n}{N}
(b) For concentrated solution (Whose mass/mass % > 5)
\tt \frac{P^{o} - P}{P^{o}} = \frac{n}{n + N}
(c) To find out molecular mass of solute for types of solutions (dilute (or) concentrated) we can use
\tt \frac{P^{o} - P}{P} = \frac{n}{n + N}
(d) Molality (m) = \tt \frac{P^{o} - P}{P} \times \frac{1000}{M(in \ gm \ mole^{-1})}
n = number of moles of solute
N = number of moles of solvent
M = Molecular mass of solvent
P0 = Vapour pressure of solvent
P = Vapour pressure of solution
(b) Ostwald walker method :
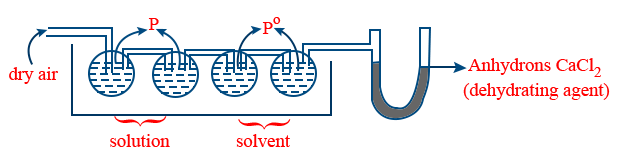
Loss in weight of solution containers α p
Loss in weight of solvent containers α (P0 − P)
Gain in weight of dehydrating agent α P0
\tt \frac{P^{o} - P}{P^{0}} = \frac{Loss \ in \ weight \ of \ solvent}{Gain \ in \ weight \ of \ dehydrating \ agent}
(ii) Elevation in boiling point:-
(a) ΔTb = kb m
where \tt \Delta T_{b} = T_{b} - T_{b}^{0}
Tb = Boiling point of solution
\tt T_{b}^{0} = Boiling point of pure liquid (solvent)
kb = Boiling point elevation constant (or) ebullioscopic constant
m = molality of solution
(b) \tt k_{b} = \frac{R(T_{b}^{0})^{2}}{1000 \ L_{v}}
Lv = Latent heat of vaporization per gram
(c) \tt k_{b} = \frac{MR(T_{b}^{0})^{2}}{1000 \ \Delta H_{vapour}}
ΔHvap = Enthalpy of vaporization per mole
M = Molar mass of solvent (in g/mol)
(iii) Depression in freezing point :-
(a) Δ Tf = kf m
where \tt \Delta T_{f} = T_{f} - T_{f}^{0}
kf = Freezing point depression constant (or) cryoscopic constant.
(b) \tt k_{f} = \frac{R(T_{f}^{0})^{2}}{1000 \ L_{f}}; Lf = Latent heat of fusion per gram
(c) \tt k_{f} = \frac{MR(T_{f}^{0})^{2}}{1000 \ \Delta H_{fus}}
ΔHfus = Enthalpy of fusion per mole
M = molar mass of solvent (in g/mol)
\tt T_{f}^{0} = Freezing point of solvent
(iv) Osmotic pressure:-(π)
(a) The hydro static pressure built up on the solution which just stops osmosis. In other words "the pressure which must be applied on the concentrated solution side to just stop osmosis"
(b) For dilute solutions π = CRT = hdg
C = concentration of solution (if must be in molarity)
R = Solution constant which is equivalent to universal gas constant
h = Height developed by the column of the concentrated solution.
Ρ = density of the solution in the column.
(c) On the basis of osmotic pressure, the solution can be classified in to three classes.
(i) Isotonic solutions:- Two solutions having same osmotic pressure are called isotonic solutions
⇒ C1 = C2 at given T
(ii) Hypertonic and hypotonic solution:-
When two solutions are being compared, then the solution with higher osmotic pressure is termed as hypertonic. The solution with lower osmotic pressure is termed as hypotonic
View the Topic in this Video from 0:26 to 1:00:46
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. The relative lowering of vapour pressure is
\tt \frac{p_1^\star-p_1}{p_1^\star}=\frac{{p_1^\star-x_1}{p_1}}{p_1^\star}=1-x_{1}=x_{2}\ or\ -\frac{\Delta\ p_{1}}{p_1^\star}=x_{2}
(where \tt \Delta p_{1}=p_{1}-p_1^\star)
2. \tt \Delta T_{b}=K_{b}m where Kb is known as boiling point elevation constant.
3. −ΔTf = Kf m where Kf is known as freezing point depression constant.
4. Osmotic pressure ∏ = cRT

