Solid State
Electrical and Magnetic Properties of Solids
- Electrical Properties:
- The solids can be conductors, insulators (or) semi conductors
- Conductors conduct electricity through movement of electrons (metals) (or) ions (molten electrolytes or aqueous electrolytes)
- The atomic orbitals of metals form molecular orbitals which are so close in energy that they form bands. If the gap between the filled valence band and the next higher unoccupied conduction band is 0eV then electrons can jump easily and are called conductors.
- If gap between valency band and conduction band is high (> 3eV) they are insulators since electrons can not jump easily from valency band to conduction band.
- If gap between valency band and conduction band is < 3eV & > 0eV i.e, ≅ 1.1eV (or) 0.72eV are called semi conductors
eg: For Si 1.1eV
Ge 0.72eV - In semi conductors, for example silicon and germanium the conductivity of these is practically less use. Therefore their conductivity can be increased by adding impurity (doping) which can be electron rich (or) electron deficient.
- When increase in conductivity is due to the electron-rich impurity, it is called n-type semi conductor.
- Group 15th elements (P, As, Sb) are electron rich impurity.
- When the increase in conductivity is due to electron-deficient impurity, the conductors are called p-type semiconductor.
- Group 13th elements (B, Al, Ga, In) are electron deficient impurities.
- If the average valency of any two elements is "4" they can be used as semiconductor.
For example compounds of Group 12 & Group 16
ZnS ⇒ Zn valency 2
S valency 6
∴ average valency = \frac{2+6}{2}=4 - With increase in temperature conductivity of conductors decreases due to increase in gap between valency band & conduction band.
- With increase in temperature in semi conductors electrons can easily jumps from valency band to conduction band so they are acting as good conductors at high temperature.
- ReO3 (Rhenium oxide) is like metallic copper in its conductivity and appearance.
- Magnetic properties:
- Paramagnetic: Substance weakly attracted by magnetic field and magnetized in same direction is known as paramagnetic substance.
- Trick for paramagnetic substance "Those having unpaired electrons in molecules".
Eg: O2, S2 gas, Cu2+, Fe2+, Cr3+ etc. - Diamagnetic: Substance weakly repelled in magnetic field is known as diamagnetic substance.
- Trick for diamagnetic substance "molecules those do not have unpaired electrons are diamagnetic"
Eg: Cu2+, Na+, C6H6, H2O etc. - Ferromagnetic: Substance attracted very strongly by magnetic field is known as ferromagnetic substance.
- Ferromagnetism can be understood by domain arrangement. The smallest magnetic region is solid is called domain. It contains approximately 1020 atoms and all of these net magnetic moments are arranged in same direction as shown below.
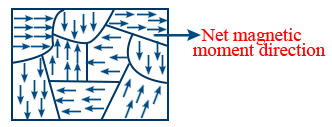
- Arrangement of domains in ferro magnetic substances
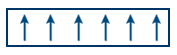
- Parallel arrangement of domains. Net μ ≠ 0.
Eg: CrO2, Fe, Co, Ni, Gd. - Anti ferromagnetic substances contain equal parallel and anti parallel arrangement of domains net magnetic moment μ = 0.
Eg: MgO 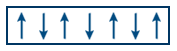
- Ferri magnetic substances contain parallel and anti parallel arrangement of domains but net magnetic moment is not equal to zero.
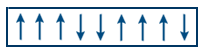
- Domain arrangement in Ferrimagnetic substances
Eg: Ferrites, Fe3O4, MgFe2O4, ZnFe2O4 etc - On heating ferromagnetic substances changes into paramagnetic substance at which temperature it is happening that temperature is called curie temperature.
Part1: View the Topic in this Video from 0:09 to 13:48
Part2: View the Topic in this Video from 0:09 to 11:34
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

