P - Block Elements
Elements and Compounds of Elements of Group-15
- VA group of periodic table consists of N, P, As, Sb and Bi having general electronic configuration ns2 np3.
These are representative elements of p-block - Due to half-filled p-orbitals (a stable configuration), these elements are not much reactive.
- All these elements show +3 oxidation state. In +3 (stable) state all elements have a tendency to donate electron pairs. This tendency is maximum in nitrogen.
- Majority of VA group compounds are covalent in nature and this tendency increases with increase in oxidation state.
| Element | Oxidation State |
| N
P As Sb Bi |
−3, −1, +1, +2, +3, +4, +5
−3, +3, +5 −3, +3, +5 −3, +3, +5 −3, +5 |
- Nitrogen shows a maximum covalency of 4 as in NH_{4}^{+} ion, because it has only four orbitals (2s and 2p) for bonding in valency shell. Other elements, due to availability of vacant d-orbitals in outermost shell are capable of showing covalency of 5 and maximum covalency of 6 as in [AsF6]− , [SbCl6]− etc.
- All these elements from trihydrides like NH3, PH3, AsH3, SbH3, BiH3. All of them acts as Lewis base. Order of basicity is SbH3 < AsH3 < PH3 < NH3.
- The trihydrides have pyramidal shape. The bond angle decrease in the order. NH3 > PH3 > AsH3 > SbH3.
- Except N2O and NO all oxides of nitrogen are acidic in nature. N2O and NO are neutral oxides. N2O (Nitrous oxide) is called laughing gas. It is prepared by heating NaNO3 and NH4Cl mixture.
- Phosphorous is an extremely active element and occurs in nature in combined state as phosphates in the rocks and in the soil.
Phosphorite - Ca3(PO4)2
Chlorapatite – 3Ca3(PO4)2. CaCl2
Apatite - 3Ca3(PO4)2 . CaF2. - Oxides of phosphorous are P4O6 and P4O10 and both are acidic in nature.
- All the pentoxides are acidic in nature and their acidic nature decreases from N2O5 to Bi2O5. The pentoxides are more acidic than corresponding trioxides.
- Oxyacid strength of nitrogen acid is H2N2O2 < HNO2 < HNO3 < HNO4
- Nitrous acid (HNO2) behaves both as oxidising agent as well as reducing agent.
- Phosphorus forms a number of oxyacids. They are
1. Phosphorous acid (H3PO4)
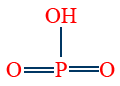
2. Meta phosphoric acid (HPO3)
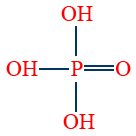
3. Ortho phosphoric acid (H3PO4)
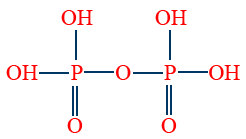
4. Pyro phosphoric acid (H4P2O7)
5. Hypo phosphoric acid (H4P2O6)
6. Hypo phosphorus acid (H3PO2)}
7. Per mono phosphoric acid (H3PO5)
8. Per di phosphoric acid (H4P2O8). - Phosphorus acid (H3PO3) is dibasic due to two – OH groups and reducing agent due to P-H bond.
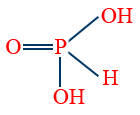
- Meta phosphoric acid (HPO3) is monobasic acid, and non-reducing agent, exists as polymer
- Ortho phosphoric acid (H3PO4) is tribasic and non-reducing.
- Pyro phosphoric acid (H4P2O7) is tetrabasic and non-reducing.
- Hypo phosphoric acid (H4P2O6) is tetrabasic and non-reducing
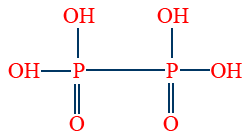
- Per mono phosphoric acid (H3PO5) is tribasic and oxidising agent.
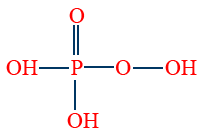
- Per di phosphoric acid (H4P2O8) is tetrabasic and oxidising agent
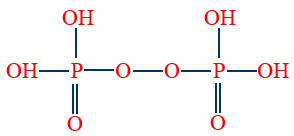
- Distinction between ortho - , meta -, and pyro phosphates.
| Reagent | Ortho phosphate | meta phosphate | pyro phosphate |
| 1. AgNO3 | Yellow ppt. | White gelatinous | White crystalline ppt |
| 2. Co(NO3)2 | Violet ppt soluble in acetic acid | no.ppt | Pink ppt insoluble in acetic acid |
| 3. Egg albumin | no action | coagulated | no reaction |
| 4. Magnesia mixture | white ppt insoluble in excess of reagent | no ppt | white ppt, soluble in excess of reagent |
- N2O is prepared by heating a mixture of NH4Cl and NaNO3. It is stable, relatively nonreactive and neutral.
- NO is formed as an intermediate in the manufacture of nitric acid by the catalytic oxidation of ammonia.
- NO is a colorless gas, paramagnetic and is very reactive.
- NO readily reacts with O2 to form reddish brown NO2 gas.
- NO is absorbed by cold FeSO4 solution to form brown colored FeSO4.NO.
- NO2 is reddish brown gas and paramagnetic.
- NO2 is obtained by heating lead nitrate. It is an odd electron molecule and is very reactive.
- N2O4 is a mixed anhydride of HNO2 and HNO3.
- N2O4 has no unpaired electron and hence it is colorless and diamagnetic.
- N2O5 is obtained by dehydration of HNO3 by P4O10. It is the anhydride of nitric acid.
- P4O6 and P4O10 are dimers. Oxygen atoms act as bridges in both the oxides. In both the oxides, number of bridge oxygen atoms is six.
- Number of oxygen atoms surrounded by ‘P’ atom in P4O6 is three and in P4O10 is four.
- NCl5 is not formed due to absence of d-orbitals in the valency shell of ‘N’
- Hydrolysis of NCl3 gives NH3 & HOCl.
- Hydrolysis of PCl3 gives HCl and H3PO3. PCl5 on hydrolysis gives HCl and H3PO4.
- For Haber's process, optimum conditions are temperature 725-775 K, pressure 200-300 atm. Finely divided iron as catalyst and molybdenum as promoter.
- Ammonia is used as refrigerant. It is used to produce fertilizers and for manufacture of HNO3.
- HNO3 obtained by Ostwald's process is about 61%. If is further concentrated by distillation until 68% is obtained distillation by mixing with conc.H2SO4 to get 98% and cooling in freezing mixture to get 100% acid.
- A mixture of conc. HNO3 and conc. H2SO4 in 1 : 1 volume ratio is called nitration mixture. It is used to convert benzene to nitro benzene.
Part1: View the Topic in this Video from 0:07 to 10:32
Part2: View the Topic in this Video from 0:07 to 8:32
Part3: View the Topic in this Video from 0:07 to 8:05
Part4: View the Topic in this Video from 0:07 to 12:20
Part5: View the Topic in this Video from 0:07 to 5:52
Part6: View the Topic in this Video from 0:07 to 5:42
Part7: View the Topic in this Video from 0:07 to 11:40
Part8: View the Topic in this Video from 0:07 to 7:27
Part9: View the Topic in this Video from 0:07 to 11:57
Part10: View the Topic in this Video from 0:07 to 14:28
Part11: View the Topic in this Video from 0:07 to 10:42
Part12: View the Topic in this Video from 0:07 to 24:39
Part13: View the Topic in this Video from 0:07 to 15:30
Part14: View the Topic in this Video from 0:07 to 9:34
Part15: View the Topic in this Video from 0:07 to 10:12
Part16: View the Topic in this Video from 0:07 to 9:53
Part17: View the Topic in this Video from 0:07 to 6:40
Part18: View the Topic in this Video from 0:07 to 10:10
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

