Haloalkanes and Haloarenes
Introduction, Methods of Preparation
- Preparation of Haloalkanes:
(a) Halogen exchange:
(i) 2 RCl (or Br) + Hg2F2 (AsF3 (or) SbF3 (or) AgF) → 2RF + H2Cl2
{Swart reaction}
(ii) \tt RCl+NaI(or \ KI)\xrightarrow[(or)Methanol]{{Acetone}}RI+NaCl
{Finkelstein reaction} 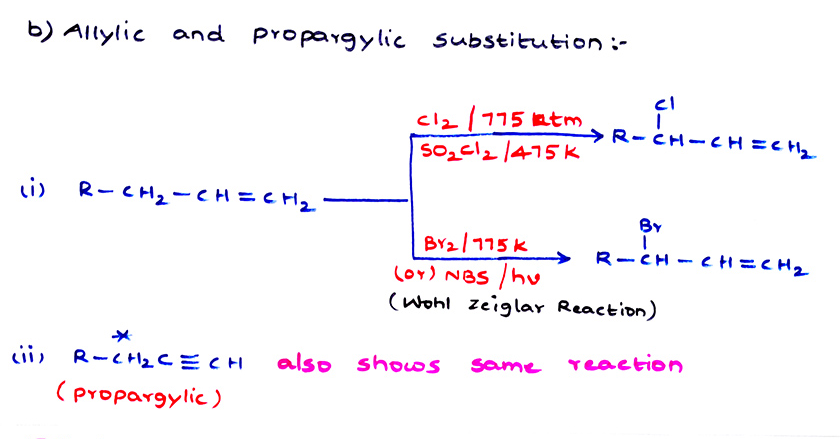
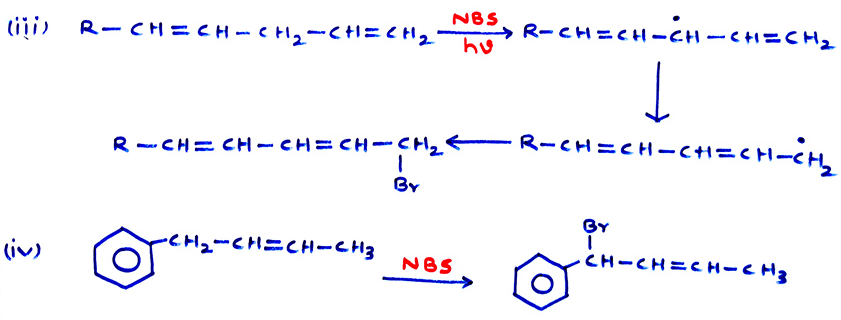
- (vii) Use of SOCl2 is the best method to convert 1° alcohols to alkyl chlorides. SO2 and HCl being gases, leave the mixture.
(viii) 3° and 2° alcohols and hindered alcohols of 1° follows SN1 mechanism.
(ix) 1° alcohols follow SN2 mechanism through 100% walden inversion.
(d) From silver salt of acid:
(i) RCOOAg + Cl2((or) Br2) → RCl + CO2 + AgX (Borodine-Hunsdiecker reaction)
(ii) 2RCOOAg + I2 → RCOOR + CO2 + 2AgI (Birnbaum Simonini reaction)
(e) From acid: 2RCOOH + 2Br2 + HgO → HgBr2 + 2R-Br + 2CO2 + 2H2O (Cristol reaction) - Preparation of Haloarenes:
-
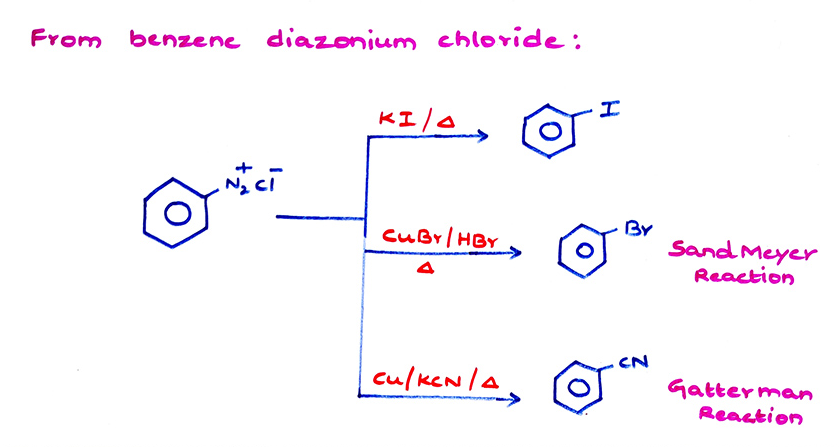
- (c) Phenol can be converted into chlorobenzene by the reaction with PCl5 but the method is not good because more than 75% Phenol is wasted in the formation of triphenyl phosphate.
C6H5OH + PCl5 → C6H5Cl + POCl3
3C6H5OH + POCl3 → (C6H5)3 PO4 + 3 HCl 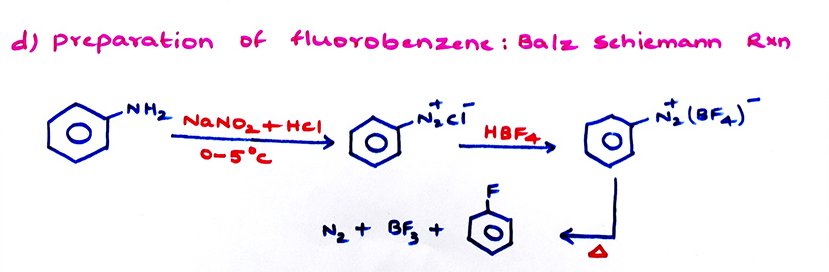
Part1: View the Topic in this Video from 0:50 to 57:05
Part2: View the Topic in this Video from 0:40 to 30:30
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

