Electrochemistry
Half - cell reactions and Cell reactions
Electrochemical cell representation and half cell and cell reaction:
(I) Metal-Metal combination:
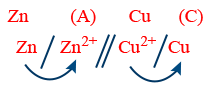
R : Anode Zn → Zn2+ + 2e−
R : Cathode Cu2+ + 2e− → Cu
Net Reaction Zn + Cu2+ → Zn2+ + Cu
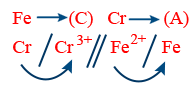
R : Anode (Cr → Cr3+ + 3e−) × 2
R : Cathode (Fe2+ + 2e− → Fe) × 3
Net Reaction 2Cr + 3Fe2+ → 2Cr3+ + 3Fe
(II) Metal-H2 combination:
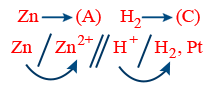
R : Anode Zn → Zn2+ + 2e−
R : Cathode 2H+ + 2e− → H2
Net Reaction Zn + 2H+ → Zn2+ + H2
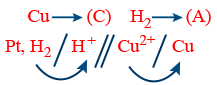
R : Anode H2 → 2H+ + 2e−
R : Cathode Cu2+ + 2e− → Cu
Net Reaction H2 + Cu2+ → 2H+ + Cu
(III) Metal Non-metal:
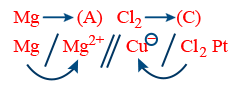
R : Anode Mg → Mg2+ + 2e−
R : Cathode Cl2 + 2e− → 2Cl−
Net Reaction Mg + Cl2 → Mg2+ + 2Cl−
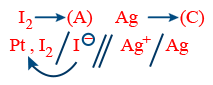
R : Anode 2I− → I2 + 2e−
R : Cathode 2Ag+ + 2e− → 2 Ag
Net Reaction 2I− + 2Ag+ → I2 + 2 Ag
(IV) Non-Metal, Non-Metal Combination:
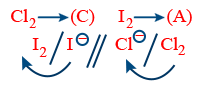
R : Anode 2I− → I2 + 2e−
R : Cathode Cl2 + 2e− → 2Cl−
Net Reaction 2I− + Cl2 → I2 + 2Cl−
Trick: In the representation of electrochemical cell we have to write Neutral to Neutral and left side oxidation always and right side reduction always.
View the Topic in this Video from 37:50 to 58:20
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

