Bio Molecules
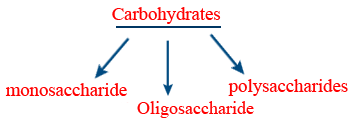
Carbohydrates
Carbohydrates :
General formula Cx(H2O)y
It is defined as an optically active poly hydroxy aldehyde or ketone or a compound which produces such units on hydrolysis.
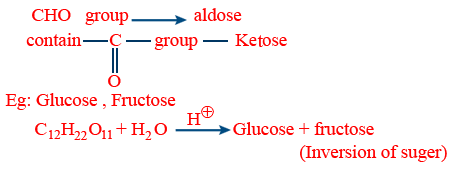
Mono saccharides : Simplest, cant be broken down by hydrolysis contains CHO group → aldose
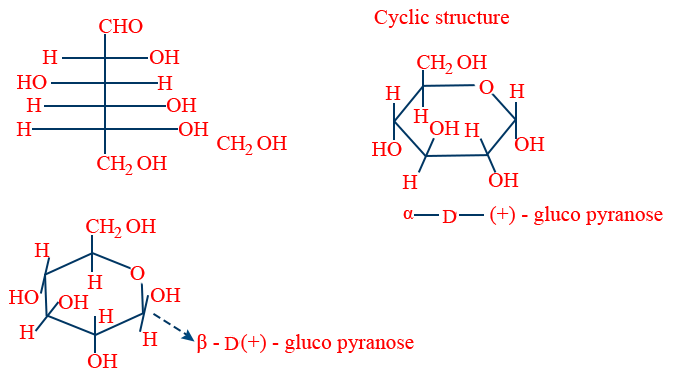
Structure of Glucose
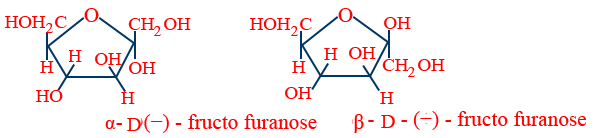
Fructose as a keto hexose :
Di saccharides : The substances which on hydrolysis give same or different mono saccharides, the units are joined by oxygen atom and the link is called glycosidic linkage.
ex : Sucrose :
It is joined by α - D - glucose and β - D - fructose joined by C1 of glucose and C2 of fructose.
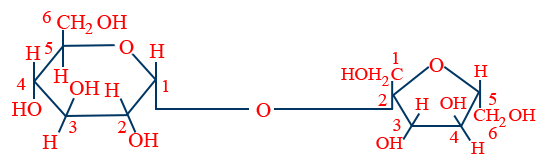
Maltose : 2(α) - D glucose link between C1 of one and C4 of other glucose.
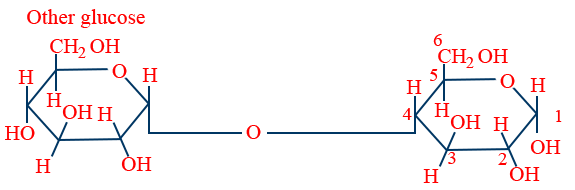
Lactose : (milk sugar) :
β - D - glucose and β - D - galactose link from C1 galactose to C4 glucose..
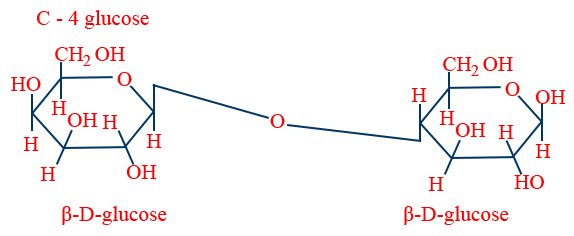
Poly saccharides :
Large no.of mono saccharide units.
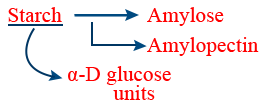
Amylose :
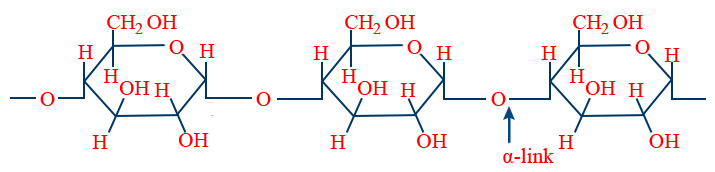
Amylopectin :
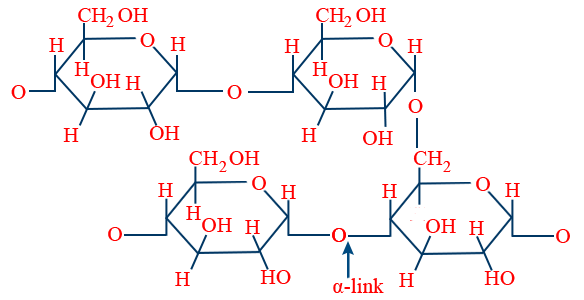
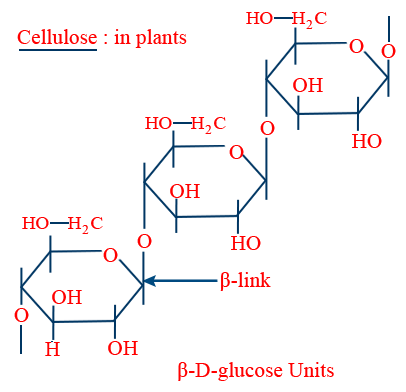
Cellulose :
Part1: View the Topic in this Video from 2:13 to 10:39
Part2: View the Topic in this Video from 0:07 to 10:45
Part3: View the Topic in this Video from 0:07 to 8:37
Part4: View the Topic in this Video from 0:07 to 6:26
Part5: View the Topic in this Video from 0:07 to 7:03
Part6: View the Topic in this Video from 0:07 to 21:04
Part7: View the Topic in this Video from 0:07 to 9:29
Part8: View the Topic in this Video from 0:07 to 13:50
Part9: View the Topic in this Video from 0:07 to 9:37
Part10: View the Topic in this Video from 0:07 to 5:03
Part11: View the Topic in this Video from 0:07 to 12:45
Part12: View the Topic in this Video from 0:07 to 15:10
Part13: View the Topic in this Video from 0:07 to 6:22
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

