States of Matter: Gases and Liquids
Liquefaction of Gases and Liquid States
Liquefaction of Gases:
| Gas | Liquid | Liquefaction takes place | |
| Attraction | Less | More | on increasing 'P' |
| KE | More | Less | on decreasing 'T' |
Inversion Temperature (Ti)
\tt T_{i}=\frac{2a}{Rb}
Ti = 2Tb
If the J-Thomson effect is done above the Ti the temperature of gas ↑
In the case of He, H2 they will get heated when they are subjected to J-T effect.
Critical Temperature (Tc):
The temperature above which a gas can not be liquefied by high pressure is known as Tc.
Critical Pressure(Pc):
The gas having high Tc can be liquefied easily.
Critical Volume (Vc):
The volume occupied by 1 mole of gas at Tc, Pc is called critical volume.
For CO2; Tc = 30.98°C
Pc = 73.9° atm
Vc = 95.6 ml/mole
Relation between Vander waal's constant (a, b) and Critical constant (Pc, Tc, Vc):
Vc = 3b, Tc = \tt \frac{8a}{27 Rb},\ Pc=\frac{a}{27 b^2}
Compressibility constant (Z) in terms of critical constant:
\tt Z=\frac{P_cV_c}{RT_C}
\tt =\frac{\frac{a}{27 b^2}\left(3b\right)}{R\cdot\left(\frac{8a}{27 Rb}\right)}\Rightarrow Z=\frac{3}{8}=0.375
Relation between Tc, Tb and Ti:
Tc : Tb : Ti = \tt \frac{8}{27}:1:2
\tt P=A\cdot e^{-\frac{\Delta W}{RT}} (P = VP)
H = (Latent heat of vaporisation)
\tt \log P=\frac{-\Delta H}{2.303\ RT}+\log X
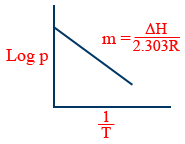
y = mx + c
\tt \log \frac{P_2}{P_1}=\frac{\Delta H}{2.303\ R}\left[\frac{1}{T_1}-\frac{1}{T_2}\right]
Surface Tension:
\tt S.T = \frac{W}{a}=J/m2 or erg cm-2
\tt =\frac{F\times l}{l\times l}\Rightarrow S.T=\frac{F}{l}
Viscosity:
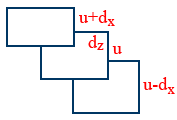
\tt F\propto A\times\frac{du}{dz}\Rightarrow F=\eta A\frac{du}{dz}
\tt \eta=\frac{F}{A}\times\frac{dz}{du}
\tt =\frac{N}{m^{2}}\times\frac{m}{ms^{-1}}=N.m^{-2}.s \Rightarrow Pa.S\Rightarrow Kg\ m^{-1}s^{-1}
Part1: View the Topic in this Video from 0:11 to 10:45
Part2: View the Topic in this Video from 37:55 to 53:40
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

