States of Matter: Gases and Liquids
Intermolecular Forces and Thermal Energy
London force (or) dispersion force: Observed in between non polar atoms or non polar molecules.
e.g. Xe and Xe
CH4 and CH4 and CCl4 and CCl4
F\propto\frac{1}{r^{6}}
Dipole-Dipole attraction: Attraction between polar compounds
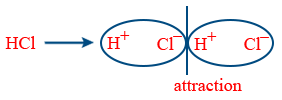
\therefore\ F\propto\frac{1}{r^{3}} → Stationary solid state
\therefore\ F\propto\frac{1}{r^{6}} → Rotational molecule
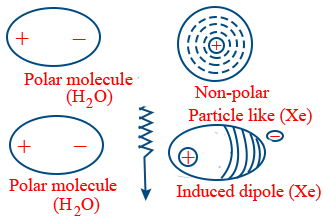
Induced dipole-dipole attraction: These are in between polar and Non-polar compounds
Ex: Solubility of inert gases in water
Volume: 1dm3 = (10 cm)3 = 1000 cc = 1000 mL = 1L
Pressure: \tt P=\frac{F}{a}
\tt P=\frac{F}{a}=\frac{mg}{a}\ \ P\Rightarrow\frac{d\times v}{a}=\frac{a\times h\times d\times g}{a}
P = hdg
In C.G.S, P = hdg, P = 1.013125 × 106 dyne/cm2
In S.I, P = hdg, 1 atm = 1.01325 Bar
Temperature \tt \frac{F-32}{9}=\frac{C}{5}
Part1: View the Topic in this Video from 2:20 to 57:11
Part2: View the Topic in this Video from 0:57 to 47:45
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. S.I unit of temperature is Kelvin (K) or absolute degree.
K = °C + 273
2. Relation between F and °C is \tt \frac{{^{o}}C}{5} =\frac{^{o}F-32}{9}
3. \tt Pressure \left(P\right) = \frac{Force\left(F\right)}{Area\left(A\right)} = \frac{Mass\left(m\right) \times Acceleration\left(a\right)}{Area\left(A\right)}
4. Absolute pressure = Gauge pressure + Atmosphere pressure

