s -Block Elements
Oxides, peroxides, hydroxides, carbonates, bicarbonates, chlorides and sulphates of sodium, potassium, magnesium and calcium
Oxides :
BeO → Amphoteric oxide
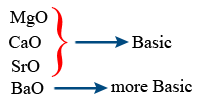
BeO < MgO < CaO < SrO < BaO → Basic nature
Hydroxides :

Ca(OH)_{2} + CO_{2} \rightarrow CaCO_{3} + H_{2}O
Ca(OH)_{2} + SO_{2} \rightarrow CaSO_{3} + H_{2}O
Stability : Increases
Solubility : Increases
Basic nature : Increases
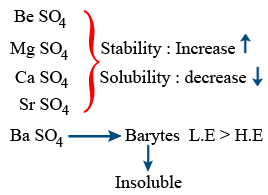
Sulphates :
Carbonates :
BeCO3
MgCO3
CaCO3
SrCO3
BaCO3
Stability : ↑ I A > II A
Solubility : ↓
BeCO3 unstable, it is kept in CO2 atmosphere to prevent decomposition.
Halides :
Fluorides :
BeF2 → soluble because more hydration energy than lattice energy
Other fluorides are insoluble
Chlorides:
BeCl2 (Vapour state)
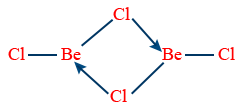
< 1200° C ⇒ Dimer
>1200° C ⇒ monomer → Cl - Be - Cl (sp hybridization)
BeCl2 (solid state)
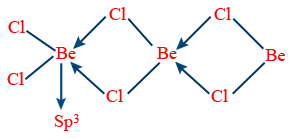
Except ' Beryllium', all other element halides are ionic.
Halides hydrated tendency decreases.
eg : MgCl2.8H2O, CaCl2.6H2O, SrCl2.6H2O, BaCl2.2H2O
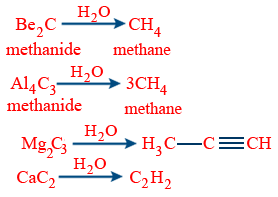
Carbides :
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

