Redox Reactions
Redox Reactions in terms of Electron Transfer Reactions
Balancing of redox reaction :
Ion electron (or) half reaction :
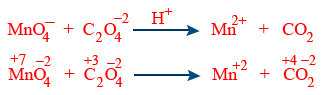
Split the reaction into two half reaction.
| LHR | RHR |
| C_{2}O_4^{2-} \rightarrow CO_{2} | MnO_4^{-} \rightarrow Mn^{+2} |
| C_{2}O_4^{-2} \rightarrow 2CO_{2} | MnO_4^{-} \rightarrow Mn^{+2} + 4H_{2}O |
| C_{2}O_4^{2-} \rightarrow 2CO_{2} | MnO_4^{-} + 8H^{+} \rightarrow Mn^{+2} + 4H_{2}O |
| C_{2}O_4^{-2} \rightarrow 2CO_{2} + 2e^{-} | MnO_4^{-} + 8H^{+} + 5e^{-}\rightarrow Mn^{+2} + 4H_{2}O |
| 5C_{2}O_4^{2-} \rightarrow 10CO_{2} + 10e^{-} | 2MnO_4^{-} + 16H^{+} + 10e^{-}\rightarrow 2Mn^{+2} + 8H_{2}O |
Finally 2MnO_4^{-} + 5C_{2}O_4^{-2} + 16H^{+} \rightarrow 2Mn^{+2} + 10CO_{2} + 8H_{2}O
II nd method (Competitive purpose)
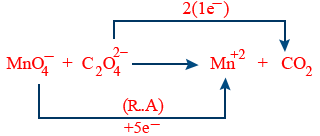
Adjust the coefficient
2MnO_4^{-} + 5C_{2}O_4^{-2} \rightarrow Mn^{+2} + CO_{2}
Balance the atoms except (O2 and H2)
2MnO_4^{-} + 5C_{2}O_4^{-2} \rightarrow 2Mn^{+2} + 10CO_{2}
Balancing O2 molecule by water (H2O)
2MnO_4^{-} + 5C_{2}O_4^{-} \rightarrow 2Mn^{+2} + 10CO_{2} + 8H_{2}O
Balancing H+ ions
2MnO_4^{-} + 5C_{2}O_4^{-} + 16H^{+} \rightarrow 2Mn^{+2} + 10CO_{2} + 8H_{2}O
View the Topic in this Video from 0:40 to 49:38
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

