p-BLOCK ELEMENTS GROUP 14(CARBON FAMILY)
Structure, properties and uses of Allotropes and Oxides of carbon
Carbon Allotropes :
i) Crystalline
- Diamond
- Graphite
- Fullerene
ii) Amorphous :
ex : Coke, Coal ---- etc
Diamond :
sp3 - hybridisation
Bond angle is 109°28'
Bond length is 1.54Å
strong covalent bonds
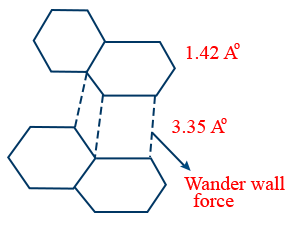
Graphite :
sp2 hybridisation
Bond length 1.4Å
Strong covalent with hexagonal rings.
Uses :
- Used as electrode
- used in lead pencil (% of lead is zero)
- Good conductor of electricity
- Thermodynamically more stable
Fullerenes : (C60)
Heating of graphite in an electric cork in the presence of inert gases such as He/Ar then sooty material formed by condensation of vapourised Cn molecules consists mainly C60 called fullerens.
Properties :
It has cage like structure
C60 shape - Soccer ball / foot ball
It contains 20 - 6 membered rings
12 - 5 membered rings
It is also called as Buckminster fullerene / Bucky ball.
View the Topic in this Video from 0:07 to 16:25
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

