p-BLOCK ELEMENTS GROUP 13(BORON FAMILY)
Structure, properties and uses of borax, boric acid, diborane, boron trifluoride, aluminium chloride and alums
Boron compounds :
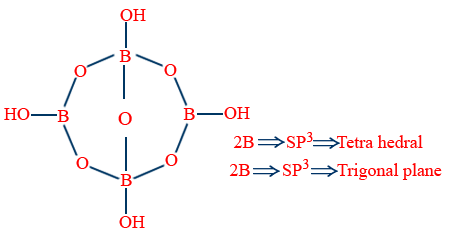
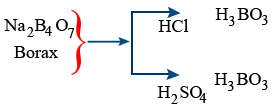
Borax (Na2B4O7 . 10H2O)
Na2[B4O5(OH)4].8H2O
Preparation :
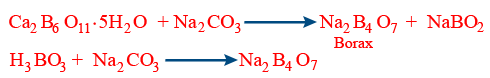
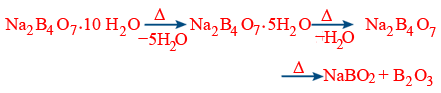
Boric Acid (H3BO3) :
BCl_{3} + H_{2} \rightarrow B_{2}H_{6}
BCl3 on silent electric discharge gives diborane
BF3 on reduction with lithium hydride gives diborane
BF3 on NaH gives diborane.
NaBH_{4} + I_{2} \xrightarrow[]{Diborane} B_{2}H_{6} + NaI + H_{2}
Chemical properties :
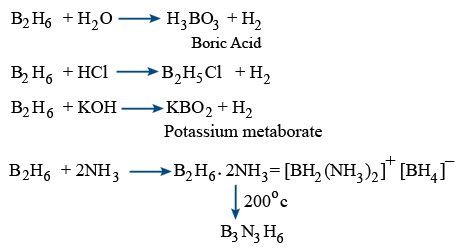
Inorganic benzene (B3N3H6) :
Similar to benzene
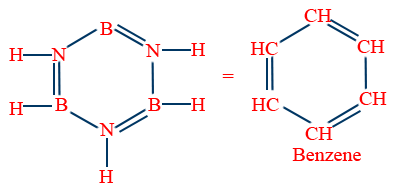
Obeys Huckel's rule
It is aromatic
12σ , 3π
B_{3}N_{3}H_{6} \xrightarrow[]{\Delta}(BN)_{x}
Boron nitride (or) Borazone
Inorganic graphite
Structure similar to graphite
Ca_{2}B_{6}O_{11} + SO_{2} + H_{2}O \rightarrow H_{3}BO_{3}
On heating H3BO3 at 100°C it gives meta boric acid (HBO3) on strong heating B2O3 (Boron oxide) at 435 Kelvin H2B4O7 on further heating B2O3 (Boron oxide)
Part1: View the Topic in this Video from 0:07 to 9:51
Part2: View the Topic in this Video from 0:07 to 7:12
Part3: View the Topic in this Video from 0:07 to 9:24
Part4: View the Topic in this Video from 0:01 to 32:53
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

