Hydrogen
Structure, Preparation, Properties and Uses of hydrogen peroxide
Concentration of H2O2 :
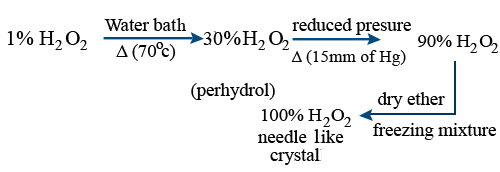
Physical properties :
Boiling point → 152º C
Melting point → −0.4º C
It is completely miscible in water.
It is stored in plastic (or) wax coated glass bottles.
Rough surface can decompose H2O2
H2O2 (Hydrogen peroxide) acts as oxidising agent and also reducing agent in both acidic and basic media.
H2O2 acts as a stronger oxidising agent than reducing agent due to high S.R.P value
Oxidising properties → \tt H_{2}O_{2} + [O], nascent \ oxygen
Acidic medium → \tt H_{2}O_{2} + 2H^{+} + 2e^{-} \rightarrow 2H_{2}O, E^{o} = +1.77 V
Basic medium → \tt H_{2}O_{2} + 2e^{-} \rightarrow 2OH^{-}, E^{o} = +0.87 V
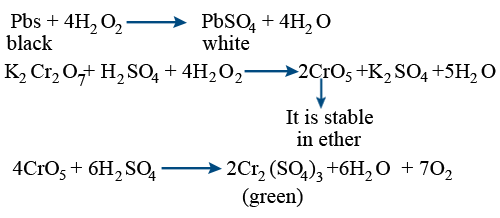
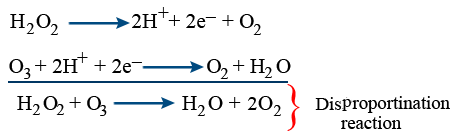
With O3
Cl2 reacts with \tt H_{2}O_{2} \rightarrow 2HCl + O_{2}(antichlor)
Bleaching action :
\tt H_{2}O_{2} \rightarrow H_{2}O + [O] nascent \ oxygen
Its bleaching action is due to nascent [O] oxygen.
Org - matter(Veg - matter coloured) + [O] → H2O + Colourless matter.
Its bleaching action is permanent.
SO2 bleaches substances by reduction (Temporary action)
Structure of H2O2 :
Open book structure
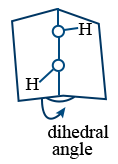
- Hybridization sp3
- Non linear, non planar
- O — H is polar
- O — O is non polar.
| Gas phase | Solid phase | |
| \angle O - OH | 94°48' | 101°5' |
| Dihedral angle | 111°30' | 90° |
| O — O | 1.48 Å | 1.485 Å |
| O — H | 0.95 Å | 0.988 Å |
Strength of H2O2 is expressed in terms of volume i.e 10 vol, 20 vol ----------
It is decided by the volume of O2 liberated.
Vol of O2 gas liberated = Vol of H2O2 × strength of H2O2 in volumes
2 lit of 10vol H2O2 liberates 20 lit of O2
5 ml of 20 vol H2O2 liberates 100 × 10−3 lit O2
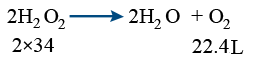
O2 liberated = 10 × 1 = 10 L of O2
68gr — 22.4 L of O2
? — 10 L of O2
x = \frac{68 \times 10}{22.4} = 30.36 \ of \ H_{2}O_{2}
= = \left\{30.36 \left(\frac{w}{v}\right) \% \right\} \ \rightarrow Perhydrol
molarity = \frac{w}{GMW \times vl}
= \frac{30.36}{34 \times 1} = 0.893 M = 0.893 × 2 = 1.786 N
Part1: View the Topic in this Video from 0:06 to 9:08
Part2: View the Topic in this Video from 0:06 to 1:46
Part3: View the Topic in this Video from 0:06 to 11:21
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. Strength of H2O2 in terms of normality
\tt \frac{68 \times X}{22.4}=17 \times N \Rightarrow X = 5.6 \times N
where, X is volume strength of H2O2.
2. % strength = \tt \frac{17}{56}\times volume\ strength
3. X = 11.2 × molarity.

