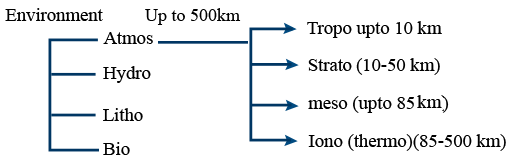
Environmental Chemistry
Atmospheric Pollution by Gaseous Pollutants
Pollutant: Substance present in nature, but conc ↑ due to human activities and cause harmful effects.
1° eg: Dust, CO2, CO, Pb, Hg, NO.... etc
2° eg: NO2, SO3.... etc
Contaminant: Substance not present in nature, but released due to human activities.
eg: MIC (1989) Bhopal tragedy
CFC (Chloro Fluoro Carbon)
DDT (Di chloro Diphenyl Trichloro ethane)
Receptor: The medium effected by the pollutant
eg: Eyes, Skin etc...
Sink: The medium which reacts with pollutants
eg: Oceans, trees etc... microorganisms
Threshold Limit value (TLV): The value upto which a person is exposed to, in shown
COD: The amount of O2 required to oxidise the organic matter
→ measure of quality of water.
Pure water 1ppm; polluted water → 17ppm
\tt COD=\frac{weight\ of\ K_{2}Cr_{2}O_{7}\left(mg\right)\times1000}{49\times weight\ of \ water\left(g\right)}\times8
BOD: The amount of O2 required by microorganism in 5 days at 20°C
It also decides the quality of water.
\tt BOD=\frac{No.of\ mg\ of\ O_{2}\ consumed}{No.of\ lit\ of\ water}
Oxides of Sulphur:
S + O2 → SO2
2SO2 + O2 → 2SO3 Particles
SO2 + O3 → SO3 + O2
SO3 + H2O → H2SO4
2SO2 + O2 + H2O → H2SO4
→ Irritation
→ respiratory problems
Oxides of nitrogen:
\tt N_{2}+O_{2}\xrightarrow{10,000^{0}C}2NO
2NO + O2 → 2NO2
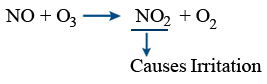
→ Harmful effects on plants
→ Respiratory problems
Acid rains pH < 5.6
→ Stratospheric
\tt CCl_{2}F_{2}\xrightarrow{UV}\dot{C}F_2+\dot{Cl}
\tt O_{3}+C\dot{l}\longrightarrow\dot{C}lO+O_{2}
\tt \dot{C}lO+O\longrightarrow\dot{C}l_2+O
1 CFC(Cl free radical) can damage 1 lakh molecules of ozone.
Summer (Antarctica) Depletion ↓
\tt CH_{4}+\dot{C}l\rightarrow \dot{C}H_{3}+HCl
\tt NO_{2}+\dot{C}lO\rightarrow ClONO_2
acts as sinks, for 'cl' free radical
Winter:
\tt ClONO_2 + H_2O\longrightarrow HOCl + HNO_3
\tt ClONO_2 + HCl\longrightarrow Cl_2 + HNO_3
Springs: \tt HClO \xrightarrow {h\nu} OH+\dot{C}l
Part1: View the Topic in this Video from 0:07 to 8:13
Part2: View the Topic in this Video from 0:07 to 22:18
Part3: View the Topic in this Video from 0:08 to 13:31
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

