Classification of Elements and Periodicity in Properties
Electronic Configurations of elements and s,p,d,f-Block Elements
s - block :
electron enters into 's' orbital
GEC = ns1 − 2
it consists I A and II A
I A - Alkali metals (GEC = ns1)
II A - Alkaline earth metals (GEC = ns2)
High electropositive metals
p - block :
the electron enters into 'p' - orbital
GEC = ns2np1 − 6
6 Groups → III A, IV A, VA, VI A, VII A and Zero groups
d - block :
In which electron enters into d - orbital
GEC = (n − 1)d1 − 10 ns1 − 2
3d - series Sc (Z = 21) — Zn (Z = 30)
4d - series Y (Z = 39) — Cd (Z = 48)
5d - series La (Z = 57) — Hg(Z = 80) * (58 - 71)
6d - series Ac (Z = 89)
f- block :
differentiating e− enters into f - orbital
GEC : (n − 2)f1 − 14 (n − 1)d0 − 1 ns2
→ Consists of two f - series
4f : Ce (58) — Lu (71)
5f : Th (90) — Lr (103)
Representative elements :
GEC : ns1 − 2 np0 − 5
In which outer most shells are incompletely filled
All 's' and 'p' block elements except noble gases
Transition elements :
General electronic configuration : (n − 1)d1 − 10 ns1 − 2
Incompletely filled outermost nth
penultimate (n − 1)
Anti penultimate (n − 2)
elements having incompletely filled are called " d - orbitals "
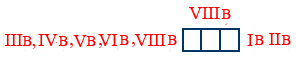
Note : except IIB group elements all d - block elements are transition.
Inner transition elements :
GEC (n − 2)f1 − 14 (n − 1)d0 − 1 ns2
Classified into two types.
4f - block elements :
Lanthanides / Lanthanoids / 4f - series / rare earths
from Ce (Z = 58) → Lu (Z = 71)
5f - block elements :
Actinides / Actinoids / 5f - series
from Th (Z = 90) → Lu (Z = 103)
→ Artificially synthesized
→ Radio active
→ Trans uranic elements - elements that come after uranium (Z = 92)
Part1: View the Topic in this Video from 0:12 to 13:23
Part2: View the Topic in this Video from 0:02 to 3:41
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. General electronic configuration of s-block elements = ns1-2.
2. General electronic configuration of p-block elements = ns2np1-6.
3. General electronic configuration of d-block elements = ns1-2(n-1)d1-10.
4. General electronic configuration of f-block elements = (n-2)f1-14 (n-1) d0-1 ns2.
5. For s-block elements; group number = number of ns-electrons (Number of valence electrons)
6. For p-block elements; group number = 10 + number of ns and np electrons
7. For d-block elements; group number = the sum of the number of (n-1)d and ns electrons
8. For f-block elements; group number is 3.

