Chemical Thermodynamics
Fundamentals of thermodynamics, System and Surroundings
System : A specified part of the (universe) which is under experimental verification.
Surrounding : Other than system is called surroundings
Boundary : Which divides system and surroundings.
Types of system :
Open system : Both energy and matter can be exchanged.
Closed system : Only energy can be exchanged it is sealed but not insulated.
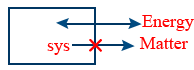
Isolated system : Either matter or energy can't be exchanged here it is sealed and isolated, the boundary is adiabatic wall
Homogeneous system : Having only one physical surface
Heterogeneous system : Having more than one physical surface.
Thermodynamic process :
Isothermal process :
Takes place at constant temperature T
ΔPH = 0
It takes place in closed system.
Adiabatic process :
In which neither energy nor matter exchanged between system and surroundings.
It takes place in isolated system.
Temperature varies (i.e ↓ or ↑)
'Q' remains constant
Isobaric process : ΔP = 0
Takes place at constant pressure
Takes place when a freely movable piston is used
volume of system varies.
Isochoric process : ΔV = 0
Takes place at constant volume
occurs when piston fixed, pressure varies.
Cyclic process :
It proceeds through no.of intermediate stages and finally reaches to the initial state. ΔE = 0
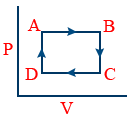
A → B → Isobaric
B → C → Isochoric
C → D → Isobaric
A → D → Isochoric
Reversible process :
Proceeds through no. of infinitely small steps and very slow and in each stage it is in equilibrium
It is an ideal process
Practically not possible
Work done is maximum in reversible process.
Irreversible process :
→ which takes place in single step.
→ work done is not maximum.
View the Topic in this Video from 1:47 to 24:49
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

