Surface Chemistry
Freundlich and Langmuir Adsorption Isotherms
- Adsorption Isobar: The graphs relating the extent of adsorption of adsorbate with temperature, at constant pressure are called adsorption isobars.
- Isoster: We can keep x/m constant by increasing temperature and pressure both the graph between temperature and pressure for a given extent of adsorption is called isoster.
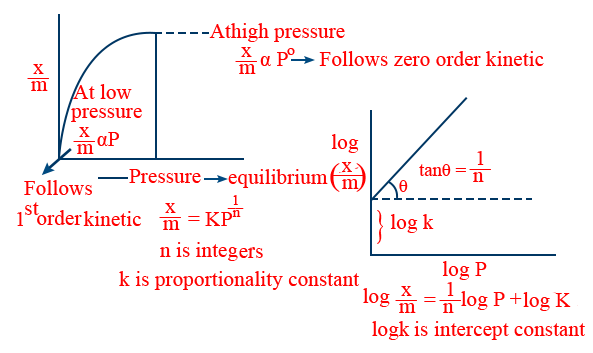
- Freundlich adsorption isotherm:(\frac{x}{m} = f(p) at constant T.)
The mathematical relations of extent of adsorption \left(\frac{x}{m}\right) with pressure at constant temperature and corresponding graphs, as studied by Freundlich are called Freundlich adsorption isotherms. - Graphs {Freundlich}:
- Langmuir adsorption isotherms:
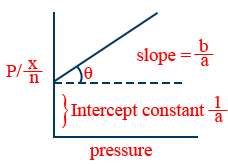
- (i)
\frac{x}{m}=\frac{ap}{1+bp}
\frac{P}{(x/m)}=\frac{b}{a}p+\frac{1}{a}
(ii) At low pressure 1+ bp ≈ 1
\therefore \frac{x}{m}=ap
\therefore \frac{x}{m}\propto p
(iii) At high pressure 1+ bp ≈ bp
\therefore \frac{x}{m}=\frac{ap}{bp}=\frac{a}{b} - Applications of adsorption:
(i) Activated charcoal is used to adsorb coloring matter from sugar-cane juice during the manufacture of cane-sugar(sucrose)
(ii) Activated charcoal is used in gas masks to adsorb poisonous gases.
(iii) In heterogeneous catalysis, gaseous reactants are adsorbed on the surface of same solid catalyst.
(iv) Moisture can be removed from a chamber by adsorbing it on silica gel.
(v) The process of chromatography is based on adsorption of substances.
(vi) Nickel is used as adsorbent for H2 gas to activate it during the hardening of vegetable oil to vegetable ghee.
(vii) Al3+ ion is tested based on the capacity of Al(OH)3 to adsorb blue litmus in "lake test" - Adsorption from solutions:
(i) Solutes can be adsorbed from solids eg: charcoal adsorbs acetic acid from solution.
(ii) The extent of adsorption decreases with increase in temperature.
(iii) The extent of adsorption increases with increase in surface area of the adsorbent.
(iv) The extent of adsorption depends upon concentration.
\frac{x}{m}=kC^{1/n}. C is equilibrium molar concentration X = Cinitial − Cfinal
(v) The extent of adsorption depends upon the nature of adsorbent and adsorbate.
View the Topic in this Video from 0:08 to 9:30
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. Freundlich Adsorption Isotherm
\tt \Rightarrow\log\frac{x}{m}=\log k+\frac{1}{n}\log p
2. Langmuir Adsorption Isotherm
\tt \frac{x}{m}=\frac{ap}{1+bp}

