Polymers
Natural and synthetic rubber and vulcanization
Rubber :
Empirical formula : C5H8
Structure :
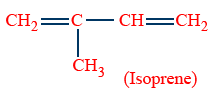
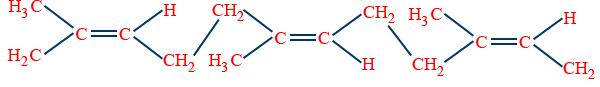
Natural rubber : Poly isoprene.
Cis isoprene formed by isoprene units which are joined head to tail by 1,4 links.
Trans - isomer : Gutta percha
Natural rubber is manufactured from rubber latex which is colloidal dispersion of rubber in water. Rubber has to be coagulated with acetic acid (or) HCOOH.
Vulcanisation of Rubber :
This process consists of heating a mixture of raw rubber with 'S' and ZnO or Zinc stearate between 373 K to 415 K. On vulcanisation, sulphur forms cross links at the reactive sets of double bonds and the rubber gets stiffened. Tyre rubber - 5% of 'S'.
View the Topic in this Video from 28:22 to 52:10
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

