Organic Compounds Containing Nitrogen
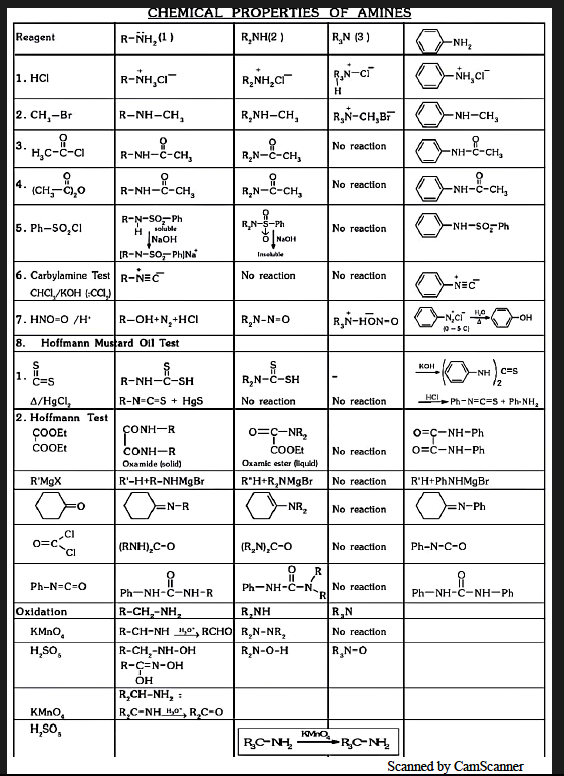
Physical and Chemical Properties of Amines
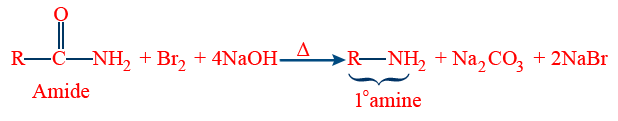
Hoffmann Bromamide Degradation Reaction
Best method for removing of carbon:
When amide is treated with Bromine in the presence of strong base gives amine which has 1 carbon less than that of substrate that's why reaction is called degradation reaction
Note:- The above method is useful in various conversions
Toluene — Aniline
PHYSICAL PROPERTIES
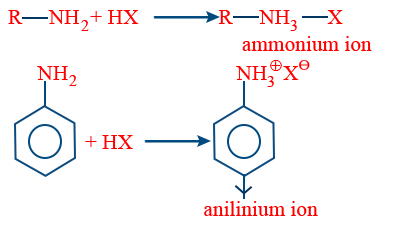
Aniline is soluble in dil. HCl because it can form aniline ions.
Amines are in general colorless due to interaction with atm. O2 they turns to brown colour.
Basic nature of amines:- When amines are treated with an acid forms salts, this inferences the basic nature of
Basic strength can be understood with reference of base dissociation constant kb or power of base dissociation constant Pkb.
According to kinetics:-
\tt R-NH_{2} + H_{2}O \rightleftharpoons R - NH_{3}^{\oplus} + OH^{\ominus}
\tt K_{equi} = \frac{\left\{R-NH_{3}^{\oplus}\right\}\left\{OH^{-}\right\}}{\left\{R-NH_{2}\right\}\left\{H_{2}O\right\}}
\tt K_{equi} \left\{H_{2}O\right\} = \frac{\left\{R-NH_{3}^{\oplus}\right\}\left\{OH^{-}\right\}}{\left\{R-NH_{2}\right\}}
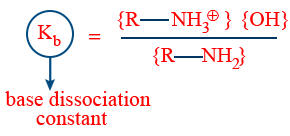
Higher the kb, ↑ the basic strength
Pkb = −log kb
lower the Pkb higher the basic nature.
In gaseous phase: -
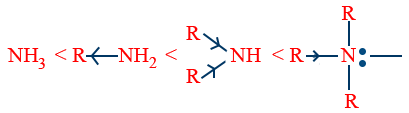
i.e, NH3 < 1° < 2° < 3°.
In case of aq. phase.
Basic strength influenced by following 3 factors:-
Solvation, Inductive effect, Steric effect.
All those three effects makes 2° amine more basic than either 1° or 3°
In case of methylamines :- [Solvation dominates + I]
(CH3)2NH > CH3NH2 > (CH3)3 N > NH3
In case of ethyl or any other aliphatic amines:-
+I effect dominates solvation.
All aliphatic amines are more basic than ammonia and aromatic amines.
Isomeric amines alicyclic amines are more basic than aliphatic
Since +I effect more in case alicyclic than aliphatic.
In case of aromatic amines the lone pair of nitrogen is always engaged with aryl group through +R effect as aryl amines are less basic than ammonia.
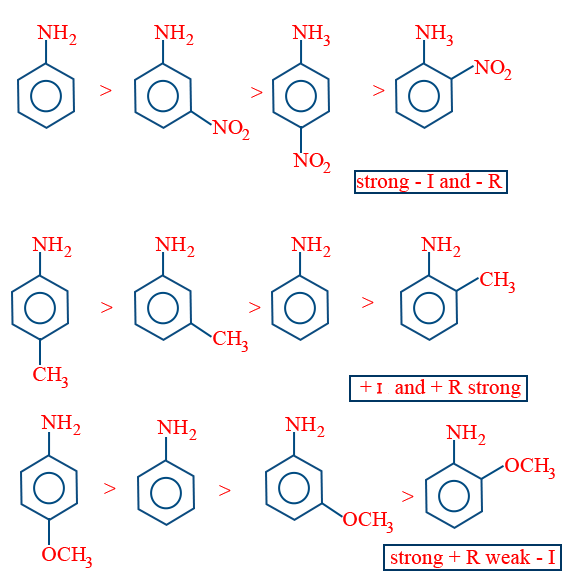
AFFECT OF GROUP TOWARDS BASIC STRENGTH OF ARYL AMINES
ORTHO EFFECT:
Whether it is e− releasing or withdrawing group present at ortho to NH4 except (ortho amino phenol) remaining all ortho substituted amines are less basic than aniline.
eΘ releasing group present at para position increases the eΘ density on nitrogen and makes it more basic than aniline.
If eΘ withdrawing group present at para position decreases the eΘ density on nitrogen makes it less basic than aniline.
The relative basic strength also influenced by inductive effect of corresponding substituent.
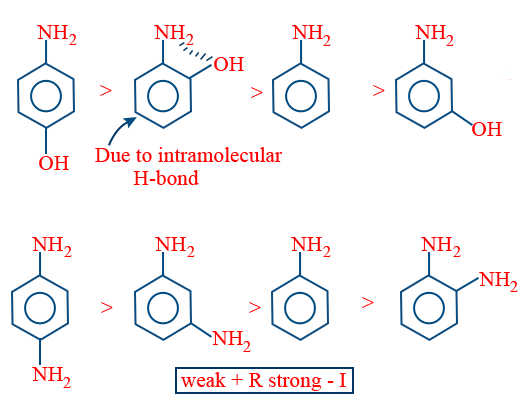
In case of ortho amino phenone due to ability to form intramolecular hydrogen bond ortho amino phenone is basic then aniline.
Special Case:
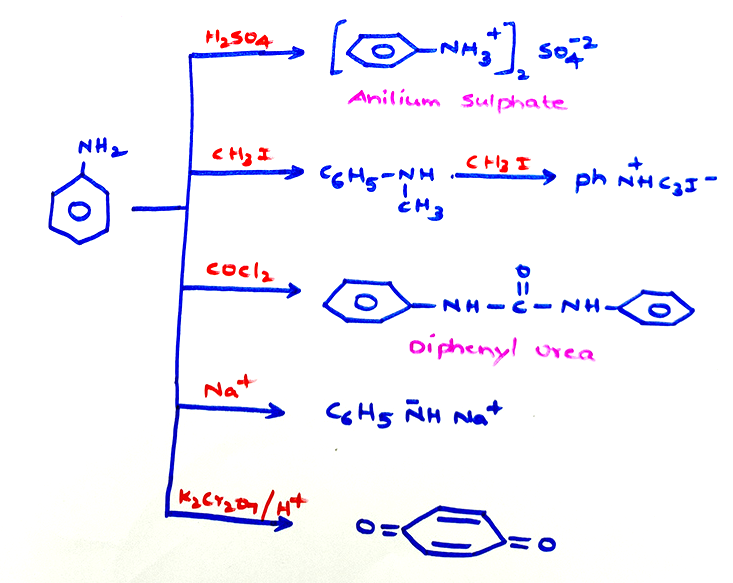
Chemical properties of aniline:
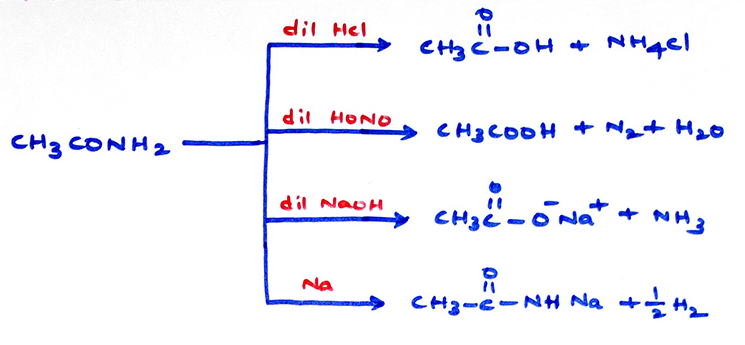
Chemical properties of amides:
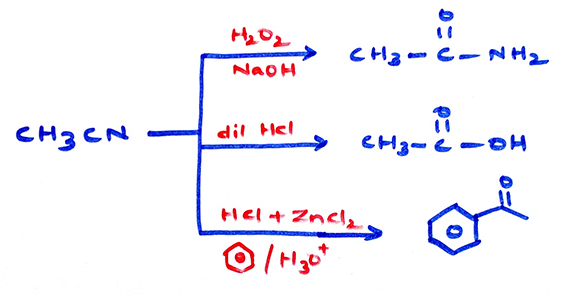
Properties of cyanides:
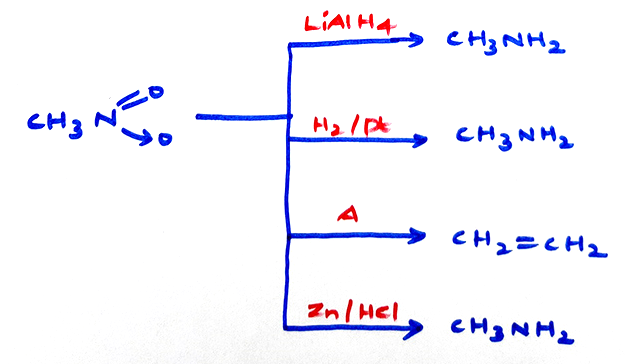
Properties of Nitro compounds:-
Part1: View the Topic in this Video from 25:05 to 41:50
Part2: View the Topic in this Video from 46:00 to 58:28
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

