Alcohols, Phenols and Ethers
Preparation and Properties of Ethers
(I) Preparation of Ethers:
From alkenes, by addition of alcohol, in presence of H2SO4 :
Here, the rearrangement of carbocation may give unexpected ether.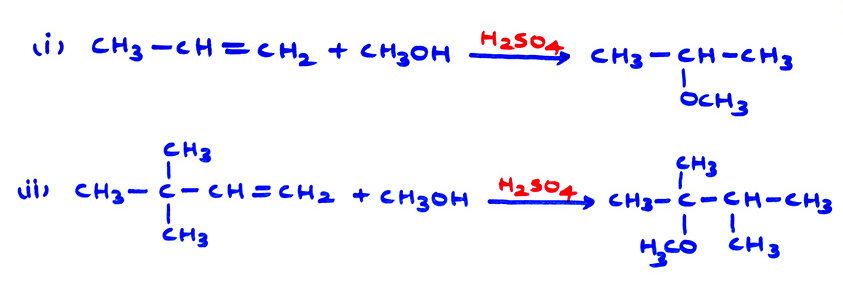
From alkylhalide:
(i) Using Ag2O
2 RCl + Ag2O → 2AgCl + R—O—R
(ii) Williamson synthesis (Main method):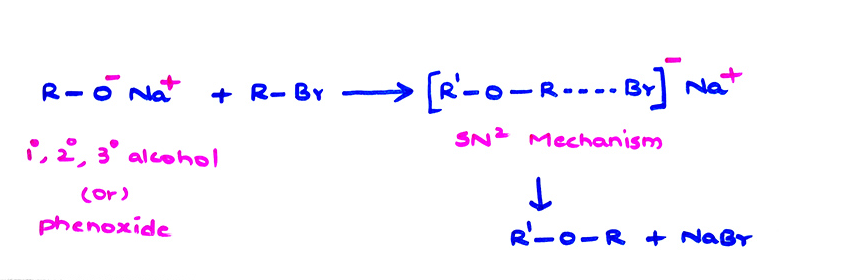
(b) H2C = CH—CH2—X react much easily because stability of allyl carbocation.
(c) 2° Alkyl halides produce both ether and alkene
(d) 3° alkyl halides produce alkene.
If t-BuOH is used in place of C2H5OH, alkene is produced as Hofmann product.
From Alcohols:
(i) Using Al2O3 at about 513 K.
\tt 2ROH\xrightarrow{{Al_2O_3/513K}}R-O-R+H_2O
(ii) Using conc. H2SO4 at 403 to 413K. [Laboratory method]
(a) 1° Alcohols follow SN2 mechanism
\tt 2C_2H_5OH\xrightarrow[130^0-140^0C]{{conc\ H_2SO_4}}C_2H_5-O-C_2H_5+H_2O
b. 2° and 3° alcohols follow SN1 mechanism forming carbocation.
c. When two different alcohols react and one is 3° alcohol, its "O" will go in the form of water because it is more basic.
e. Cyclic ethers are generally prepared by Intra molecular Williamson synthesis.
Some cyclic ethers are 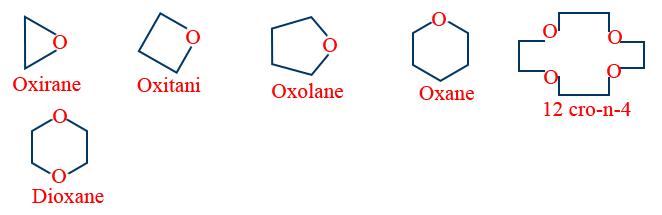
Properties of ethers:
a. These have low polarity, boiling points almost same as that of comparable molecular masses but much lower than isomers alcohols.
b. Chemical properties: Because of no special group, these are generally considered inert.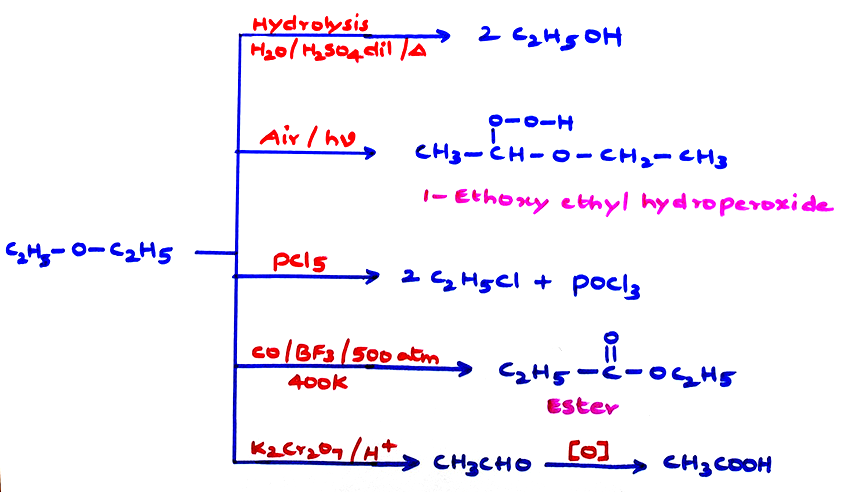
\tt \left(CH_3\right)_3C-O-C\left(CH_3\right)_3\xrightarrow[25^0C]{{HCl\ conc}}2\left(CH_3\right)_3C-Cl+H_2O
This reaction with conc. HI/373 K converting CH3O — (or) C2H5 — O — groups to CH3I (or) C2H5I is used to estimate methoxy and ethoxy groups by zeisel method, where in the iodo products are further reacted with ethanolic AgNO3. The precipitates of AgI are dried and weighed
No. of methoxy groups = \tt \frac{wt. of\ AgI}{wt\ of\ ether}\times\frac{31(mass\ of\ CH_3O)}{235(molar\ mass\ of\ AgI)}
Any old sample of ether may contain peroxide group which causes explosion on heating such a sample, before using is tested FeSO4 followed by KCNS that gives blood red colour.
Peroxide as ether + FeSO4 + H2SO4 → Fe2(SO4)3 + H2O + normal ether
Fe3+ + 3CNS− → Fe(CNS)3 (Blood red)
Excess of FeSO4 / H2SO4 removes the total peroxide and the normal ether can be used in experiment.
Part1: View the Topic in this Video from 0:08 to 19:32
Part2: View the Topic in this Video from 0:08 to 2:17
Part3: View the Topic in this Video from 0:08 to 20:44
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

