Structure of Atom
Quantum Numbers and Electronic Configuration of Elements
Quantum Numbers :
- a. Principal quantum number (n)
- b. Azimuthal quantum number (l)
- c. Magnetic quantum number (m)
- d. Spin quantum number (s)
a. Principal quantum number (n)
r_{n} = 0.54\frac{n^{2}}{Z}
Indicates size of the orbit.
Indicates energy of electron
b. Azimuthal quantum number (l)
Denoted by letter ' l ' and can have value from 0,1 to (n − 1)
l = 0 → s orbital → spherical
l = 1 → p orbital → dumb-bell
l = 2 → d orbital → double dumb-bell
l = 3 → f orbital → fore fold
c. Magnetic quantum number (m)
can have values −l to +l
total values (2l + 1)
d. Spin quantum number (s)
It can have values s = +½ and s = −½
Exchange Energy :
Exchange Energy = nC2
=\frac{n!}{(n - 2)! \ 2!}
n = no.of unpaired electrons
Cr = 3d5 4s1
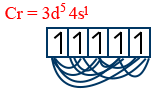
n = 6
6C2 = 15
Magnetic \ moment (\mu) = \sqrt{n(n + 2)} BM
| n | μ=\sqrt{n(n + 2)} BM |
| 1 | \sqrt{3}=1.732 BM |
| 2 | \sqrt{8}=2.823 BM |
| 3 | \sqrt{15}=3.89 BM |
| 4 | \sqrt{24}=4.88 BM |
| 5 | \sqrt{35}=5.89 BM |
Electronic configuration :
nlx method
moeller diagram :
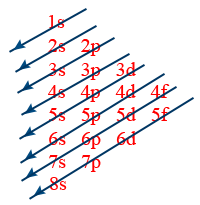
1s < 2s < 2p <3s <3p < 4s < 3d < 4p < 3s < 4d < 5p < 6s < 4f < < 5d < 6p < 7s < 5f < 6d < 7p < 8s
Part1: View the Topic in this Video from 37:35 to 50:55
Part2: View the Topic in this Video from 1:17 to 18:35
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. The number of electrons in subshell = 2(2l + 1).
2. Number of orbitals in main energy level = n2.
3. Maximum number of electrons in nth shell = 2n2
4. The orbital angular momentum of an electron = \tt \sqrt{l\left(l + 1\right)}\frac{h}{2\pi}
5. Spin angular momentum = \tt \sqrt{s\left(s + 1\right)}\frac{h}{2\pi}
6. Magnetic moment = \tt \sqrt{n\left(n + 2\right)} B.M. (Bohr magneton) of n unpaired e-

