s -Block Elements
Preparation and properties of sodium carbonate, sodium hydroxide and sodium hydrogen carbonate
Preparation of sodium hydroxide :
Nelson's cell
Castner–Kellner process
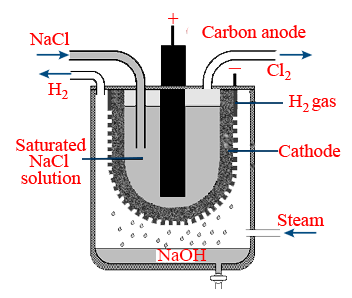
Nelson cell method :
It is a 'U' shaped perforated steel vessel
Cathode : steel vessel
Anode : graphite rod
At anode : 2Cl− → Cl2 + 2e−
At cathode : 2Na+ + 2e− + 2H2O → 2NaOH + H2 ↑
Castner - Kellner process :
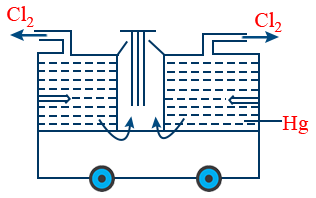
Outer compartment :
Cathode - 'Hg'
Anode - Graphite
electrolyte - Brine solution
At anode : 2Cl− → Cl2 + 2e−
At cathode : 2Na+ + 2e− + Hg → Na2Hg
Middle compartment :
Cathode - Fe rod
Anode - Hg
electrolyte - dil NaOH
At anode : Na2Hg → 2Na+ + 2e− + Hg
At cathode : 2Na+ + 2e− + 2H2O → 2NaOH
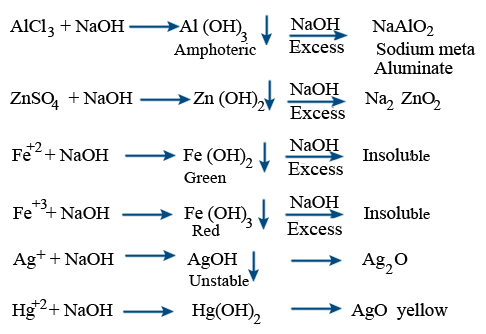
Chemical properties of NaOH :
Non metals : Cl2, Br2, I2 reacts with dil and cold, conc and hot NaOH. It gives NaOCl and NaClO3 (sodium oxychloride, sodium chlorate)
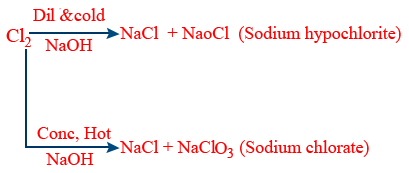
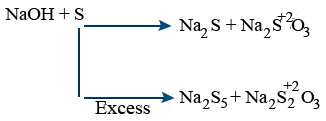
Chlorine, bromine, iodine, phosphorus gives disproportionation reaction.
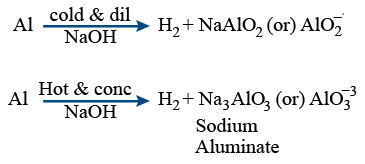
With metals :
Aluminium reacts with cold and dil. NaOH gives NaAlO2
Hot and conc NaOH gives H2 + Na3AlO3
Preparation of sodium peroxide (Na2O2)
Preparation : Na_{2}O \xrightarrow[]{\Delta}Na_{2}O_{2}

Na_{2}O_{2} + CO \rightarrow Na_{2}O_{3}
Na_{2}O_{2} + CO_{2} \rightarrow Na_{2}CO_{3} + \frac{1}{2}O_{2}
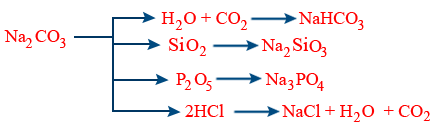
Preparation of Na2CO3 (Sodium carbonate)
Washing soda - Na2CO3.10H2O
soda ash - Na2CO3
It is prepared by 1) Leblanc process 2) Solvay process
Leblanc process : Raw materials : NaCl, Coke, limestone
2NaCl + H_{2}SO_{4}\rightarrow Na_{2}SO_{4} + 2HCl
Na_{2}SO_{4} + 4C \rightarrow Na_{2}S + 4CO

Solvay process :
NH_{3} + H_{2}O + O_{2} \rightarrow NH_{4}HCO_{3}
NH_{4}HCO_{3} + NaCl \rightarrow NaHCO_{3}\downarrow + NH_{4}Cl
2NaHCO_{3} \xrightarrow[]{\Delta} Na_{2}CO_{3} + H_{2}O + CO_{2}
2NH_{3}+ H_{2}O + CO_{2} \rightarrow (NH_{4})_{2}CO_{3}
(NH_{4})_{2}CO_{3} + MgCl_{2} \rightarrow MgCO_{3}\downarrow
Physical properties :
Efflorescent substance i.e, it looses its crystalline water molecules when it is kept in open air.
Chemical properties :
Preparation of sodium bicarbonate (NaHCO3) :
Na_{2}CO_{3} + H_{2}O + CO_{2} \rightarrow 2NaHCO_{3}
Chemical properties :
2NaHCO_{3} \xrightarrow[]{\Delta} Na_{2}CO_{3} + H_{2}O + CO_{2}
CO_3^{-2} \xrightarrow[]{H_{2}O} 2OH^{-}
HCO_3^{-} \xrightarrow[]{H_{2}O} OH^{-}

It is a mild antiseptic for skin infections and fire extinguishers.
General properties of IA group - element :
Atomic size : (↑)
down the group increases
Density : (↑)
But Na > K
Melting and boiling point : down the group decreases.
Conductivity : In free state
Li+ > Na+ > K+ > Rb+ > Cs+
Aqueous state : Li+ < Na+ < K+ < Rb+ < Cs+
Lithium has more hydrated energy.
Ionisation enthalpy down the group decreases
Hydration enthalpy down the group increases
Metallic radius down the group increases
Ionic radius down the group increases.
Reducing nature :
Due to small size lithium has more hydration enthalpy and also high negative electrode potential value.
Lithium good reducing agent → More hydration energy.
flame colourisation :
Li → Crimson red
Na → Golden yellow
K → Pale violet
Rb → Red, violet
Cs → Violet
Reactivity towards air :
M + O_{2} \rightarrow M_{2}O(oxide) \xrightarrow[]{H_{2}O}MOH \xrightarrow[]{CO_{2}}M_{2}CO_{3}
4Na + O_{2} \rightarrow 2Na_{2}O
2Na + O_{2} \rightarrow Na_{2}O_{2}(Peroxide)
4Li + O_{2} \rightarrow 2Li_{2}O(oxide)
M + O_{2}(Excess) \rightarrow MO_{2}(superoxide), M = K, Rb, Cs
2M + 2H_{2}O\rightarrow 2M^{+} + 2OH^{-}+ H_{2}
2M + H_{2} \xrightarrow[]{673 K} 2M^{+} + H^{-}
Reactivity with NH3
Aqueous NH3 :
M + NH_{3} \rightarrow Ammoniated \ ions+ Ammoniated \ electrons
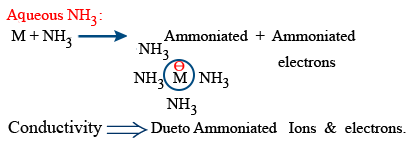
Conductivity ⇒ Due to Ammoniated ions and electrons.
Alkali metals dissolve in NH3 giving deep blue colour solution.
On warming blue colour changes to bronze colour and becomes diamagnetic.
Li2CO3 on heating gives Li2O and CO2
Li_{2}CO_{3} \xrightarrow[]{\Delta} Li_{2}O + CO_{2}
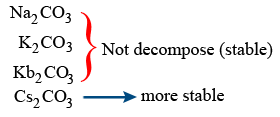
Remaining other all carbonates not decomposed by heating.
Part1: View the Topic in this Video from 0:08 to 3:55
Part2: View the Topic in this Video from 0:06 to 11:14
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

