Redox Reactions
Concept of Oxidation and Reduction
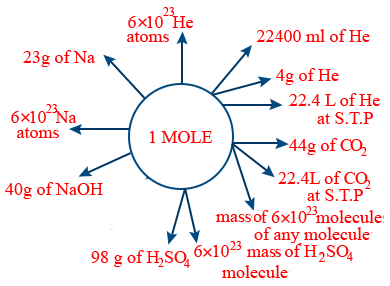
1 amu = 1.66 × 10×-24 gm
amu = \frac{1}{12} \times \frac{12}{6\times10^{23}} = 0.166 \times 10^{-23}g
1 amu = \frac{1}{N_{A}}
Limiting reagent (L.R) :
The reactant of a reaction which reacts completely
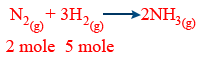
According to balanced reactions
1 mole N2 → 3mole H2
2mole N2 → 6 mole H2
L.R = H2
3 mole H2 → 1mole N2
5mole H2 → 5/3 mole of N2
mole of N2 remained unreacted = 2 − 5/3
= 1/3
Mole of NH3 formed :
3mole H2 → 2mole of NH3
5mole of H2 → \frac{5 \times 2}{3} = \frac{10}{3} = 3.33
% of purity :

100 gram — 22.4
9 gram — \frac{9 \times 22.4}{100}
[Since wt of CaCO3
\frac{10 \times 90}{100} = 9 \ gram]
\tt \% \ of \ purity = \frac{wt. of \ pure \ compound}{wt. of \ impure \ compound} \times 100
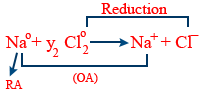
Oxidising agent (or) oxidant :
Which undergoes reduction i.e gain of e−
Reducing agent (or) reductant :
Which undergoes oxidation (or) which loses electron
Types of Redox Reactions :
Combination Reaction :

E.wt of 'C' = \frac{12}{4} = 3g
E.wt of 'O' = \frac{16}{2} = 8g
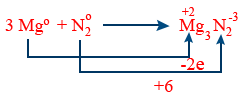
E.wt of Mg = \frac{24}{2} = 12g
E.wt of N_{2} = \frac{14}{3}g
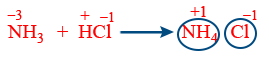
Note : Neutralisation reactions are not redox reaction.
Decomposition reaction :
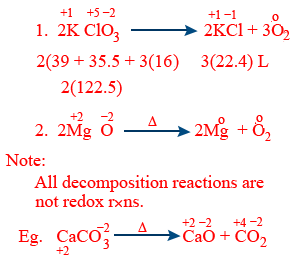
Displacement reactions :
Metal displacement reactions
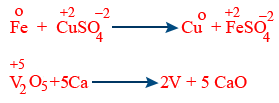
Non - metal displacement reactions :
F2 + 2KCl → 2KF + Cl2
F2 + 2KBr → 2KF + Br2
F2 + 2KI → 2KF + I2
Disproportional reactions :
Note : {Halogen, P, S are disproportional}
In this reaction the same substance undergoes both oxidation and reduction.
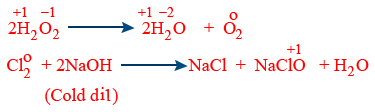
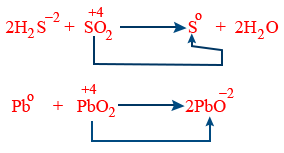
Comproportional reactions :
View the Topic in this Video from 0:40 to 46:40
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. Eq. wt. of oxidant/reductant = \tt \frac{molar \ mass}{change \ in \ oxidation \ number}
2. For disproportionation reaction,
Eq. wt. of oxidant/reductant = sum of eq. wt. of two half reactions
3. Eq. wt. of O.A. = \tt \frac{molecular \ weight}{No. \ of \ electrons \ gained \ by \ one \ molecule}
4. Eq. wt. of R.A. = \tt \frac{molecular \ weight}{No. \ of \ electrons \ lost \ by \ one \ molecule}

