Hydrogen
Physical and Chemical properties of water and heavy water
Water :
Water has high thermal capacity, high heat of vaporization, and hydrogen bond.

→ Angular / V -shape / bent shape
→ sp3 hybridization
→ 2 lone pair and 2 bond pairs.
Ice :
At atmospheric pressure, has hexagonal shape at very low temperature, it has cubical structure.
In polar region, in rivers, the aquatic life is surviving due to thermal isolation property of water.
Physical properties :
| H2O | D2O | |
| max. density | 4°C | 116°C |
| Boiling point | 100°C | 101.4°C |
| Melting point | 0°C | 3.8°C |
| molecular weight | 18 | 20 |
| Dielectric constant | 78.39 | 78.06 |
| Viscosity | less | more |
Amphoteric nature :
NH_{3} + H_{2}O \rightleftharpoons NH_4^+ + OH^{-}
HCl + H_{2}O \rightleftharpoons H_{3}O^{+} + OH^{-}
Autoprotolysis :
H_{2}O + H_{2}O \rightleftharpoons H_{3}O^{+} + OH^{-}
D_{2}O + D_{2}O \rightleftharpoons D_{3}O^{+} + OD^{-}
Hydrates formation :
| H - bonded water (hydrates) | interstitial water |
| CuSO4.5H2O | BaCl2.2H2O |
| [Cu(H_{2}O)_{4}]^{+2}SO_4^{-2}H_{2}O | BaCl2.2D2O |
Co - ordinated water :
CrCl3.6H2O
[Cr(H2O)6]Cl3
[Cr(H_{2}O)_{6}]^{+3}3Cl^{-}
Hard water : Which does not give lather (foam) with soap
Soft water : Which gives lather with soap.
Hardness :
Permanent hardness : due to Cl− and SO_4^{-2} of Ca and Mg
Temporary hardness : due to bicarbonates of Ca and Mg.
By using washing soda (Na2CO3.10H2O) both temporary and permanent hardness can be removed.
Temporary hardness :
Na_{2}CO_{3} + Ca(HCO_{3})_{2} \rightarrow CaCO_{3}\downarrow + \ 2NaHCO_{3}
Permanent hardness:
Na_{2}CO_{3} + MCl_{2} \rightarrow MCO_{3}\downarrow + \ 2NaCl \ (M = Ca^{+2} \ or \ Mg^{+2} )
Na_{2}CO_{3} +MSO_{4} \rightarrow + 2Na_{2}SO_{4} + MCO_{3}\downarrow
Calgon method : (sodium hexa meta phosphate)

Mg^{+2} + Na_{2}\left[Na_{4}(PO_{3})_{6}\right]\rightarrow Na_{2}\left[Na_{2}M(PO_{3})_{6}\right] + 2Na^{+}
Ca^{+2} + Na_{2}\left[Na_{4}(PO_{3})_{6}\right]\rightarrow Na_{2}\left[Na_{2}Ca(PO_{3})_{6}\right] + 2Na^{+}
Synthetic Resin Method :
Ion Exchange Method :
↓
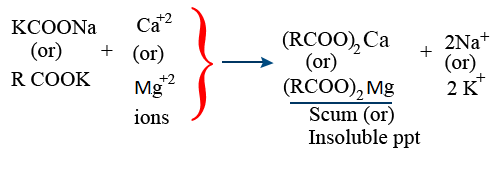
Cation Exchange Resin :

Ca^{+2} + 2RCOOH \rightarrow (RCOO)_{2}Ca + 2H^{+}
Mg^{+2} + 2RSO_{3}H \rightarrow (RSO_{3})_{2}Ca + 2H^{+}
Anion Exchange Resin : can be revived by using dil NaOH / Na2CO3
Cl^{-} + RNH_{3}OH \rightarrow RNH_{3}Cl + OH^{-}
SO_4^{-2} + 2RNH_{3}OH \rightarrow R(NH_{3})_{2}SO_{4} + 2OH^{-}
Calculation of degree of hardness of water :
Equivalents of salts of Ca and Mg are given below.
| CaCO3 = 100 g | Mg(HCO3)2 = 146 g | Ca(HCO3)2 = 162 g |
| CaSO4 = 136 g | MgSO4 = 120 g | CaCl2 = 111 g |
| MgCl2 = 95 g |
\tt Hardness \ in \ ppm = \frac{weight \ of \ salt}{mol \ wt \ of \ salt} \times \frac{100}{wt \ of \ H_{2}O \ in \ gram} \times 10^{6}
Uses of D2O (Deuterium oxide)
- used a moderator (or) coolant in nuclear reaction
- As a traces used in reaction mechanism
- used as coolant in nuclear power plants.
Part1: View the Topic in this Video from 0:05 to 6:26
Part2: View the Topic in this Video from 0:06 to 3:55
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

