Equilibrium
Le Chatelier's Principle and Factors Effecting Equilibrium
Le Chatelier's Principle :
Applicable for only reversible reaction
Effect of concentration :
If concentration of reactant ↑ / product ↓ → forward reaction
If concentration of reactant ↓ / product ↑ → backward reaction
Effect of pressure :
Pressure(P) ↑ no. of moles ↓ (or) volume ↓
Pressure(P) ↓ no. of moles ↑ (or) volume ↑
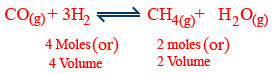
ex :
↑ of P → favours forward reaction
↓ of P → favours backward reaction
Effect of temperature :
↑ of temperature → favours endothermic reaction.
↓ of temperature → favours exothermic reaction.

ex :
↑ T → B.R → Reddish brown ↑
↓ T → exothermic → intensity RB ↓
Haber's process :
N_{2(g)} + 3H_{2(g)} \rightleftharpoons 2NH_{3(g)} ; \Delta H = -92kJ
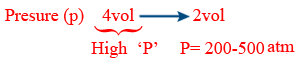
Temperature (T) : Low T
∴ 725 - 775 K
Catalyst : Fe (or) FeO
promoter :
K_{2}O + Al_{2}O_{3} (or) Molybdenum
Contact process :
2SO_{2(g)} + O_{2(g)} \rightleftharpoons 2SO_{3(g)} ; \Delta H = -189kJ
Pressure : High pressure 1 - 2 atm
Temperature : Optimum temperature, 673 - 723 K
Catalyst : Pt (or) V2O5
Degree of dissociation (α) using vapour density :
\alpha = \frac{No. of \ particles \ dissociated}{No.of \ particles \ taken}
\frac{D}{d} = 1 + (n - 1)x \ \Rightarrow \frac{D}{d} - 1 = (n - 1)x
\frac{D - d}{(n - 1)d} = x \ (or) \ \frac{M - m}{m(n - 1)} = x
D = initial vapour density
d = equilibrium vapour density.
View the Topic in this Video from 0:11 to 6:23
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. Degree of Dissociation \alpha = \frac{D - d}{d(y - 1)}
where, y = number of moles of product from one mole of reactant,
D = theoretical vapour density and
d = observed vapour density
2. \alpha = \frac{M_{c} - M_{o}}{M_{o}}
where, Mc = calculated molecular weight and
Mo = observed molecular weight.

