Equilibrium
Acids and Bases and their Ionisations
Strong acid : 100% dissociation
HClO4, H2SO4, HNO3, HI, HBr
HCl + H_{2}O \rightarrow H^{+} + Cl^{-}
Strong base :
NaOH, KOH, RbOH, CsOH, Ba(OH)2
Weak acid : Which undergoes partial dissociation
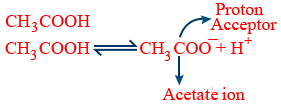
Hydrogen ion concentration :
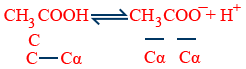
K_{a} = \frac{C{\alpha} \ C{\alpha}}{C - C{\alpha}} = \frac{C\alpha^{2}}{1 - \alpha}
Ka = Cα2 (α < < < 1, 1 − α = 1)
\alpha = \sqrt{\frac{K_{a}}{C}}
[H^{+}] = C\alpha = C. \sqrt{\frac{K_{a}}{C}} = \sqrt{K_{a}.C}
[H^{+}] = C\alpha = \sqrt{K_{a}.C}
Arrhenius acid - base theory :
Strong acid : Which produce more no. of H+ (HClO4, H2SO4, HCl)
Weak acid : Which produce less no. of H+(CH3COOH, HCN, H2S)
Strong base : Which produce more no. of OH− (NaOH, KOH)
Bronsted - Lowry acid - base theory :
Acid : Proton donor [HCl , H2SO4, CH3COOH ......]
Base : Proton acceptor[NaOH, KOH, NH3 .......]
Salt : Neither proton acceptor nor proton donor. [C6H6, CCl4 ..... aprotic]
Conjugate acid - base pair :
A Bronsted - Lowry acid - base pair which differ by only one proton is called conjugate acid - base pair.
Acid − H+ → conjugate base
Base + H+ → conjugate acid.
Ionic product of water : (KW)
H_{2}O + H_{2}O \rightleftharpoons H_{3}O^{+}+ OH^{-}
K = \frac{[H_{3}O^{+}][OH^{-}]}{[H_{2}O]^{2}}
K{[H_{2}O]^{2}} = [H_{3}O^{+}][OH^{-}]
K_{W} = [H_{3}O^{+}][OH^{-}]
Kw → acid, ΔT = 25°C
Kw = 1.0 × 10-14 mol2 lit-2
[H+][OH-] = 10-14, [H+] = 1.0 × 10-7 mol/lit
Degree of dissociation :
\alpha = \frac{1}{55.5 \times 10^{7}} = \frac{10^{-7}}{55.5} = \frac{10^{-7}}{\left(\frac{1000}{18}\right)}
α = 1.8 × 10-9
% dissociation = 1.8 × 10-7 , \alpha = \frac{10^{-7}}{55.5}
Part1: View the Topic in this Video from 0:10 to 5:20
Part2: View the Topic in this Video from 0:10 to 19:00
Part3: View the Topic in this Video from 0:10 to 9:04
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.
1. Degree of ionisation :
\tt \alpha = \frac{number \ of \ molecules\ ionised \ or \ dissociated}{total \ number \ of \ molecules \ taken}
For strong electrolytes, α = 1
For weak electrolytes α < 1
2. Ostwald's Dilution law :
k = \frac{C\alpha^{2}}{1 - \alpha}
If α is very small 1 - α ≈ 1 ⇒ K = Cα2
or \alpha = \sqrt{\frac{K}{C}} \Rightarrow \alpha \propto \frac{1}{\sqrt{C}}
Here, K is dissociation constant and C is molar concentration of the solution.
3. Dissociation constant of acid, K_{a} = \frac{\left[H^{+}\right]\left[A^{-}\right]}{\left[HA\right]} =\frac{C\alpha^{2}}{\left(1 - \alpha\right)}
4. Dissociation constant of the base K_{b} = \frac{\left[B^{+}\right]\left[OH^{-}\right]}{\left[BOH\right]} =\frac{C\alpha^{2}}{\left(1 - \alpha\right)}

