Classification of Elements and Periodicity in Properties
Valency, Oxidation states and Chemical reactivity
Oxidation State / Oxidation Number :
The amount of charge appears to be gained by an atom in a molecule is called oxidation state.
ex : O-2, F-, N-3, S-2 -----
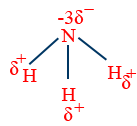
Inert pair effect :
s- orbitals behave like inert gas because it is strongly attracted by nucleus cause poor shielding
→ Thallium = 6s2 6p1
Tl+ = stable
Down the group, oxidation state decreases so more stable.
Pb+2 > Pb+4 (stability)
Diagonal relationship :
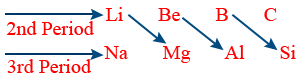
element of a group in 2nd period resembles in its properties.
Reasons for diagonal relationship :
→ Nearly same atomic and ionic size
→ Nearly same electronegativity
→ Nearly same polarising power.
\tt Polarising \ power = \frac{Ionic \ character}{(Ionic \ radius)^{2}}
Be and Al are amphoteric.
Part1: View the Topic in this Video from 0:11 to 11:03
Part2: View the Topic in this Video from 0:12 to 11:52
Part3: View the Topic in this Video from 0:16 to 6:37
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

