Chemical Bonding and Molecular Structure
Valence Bond Theory and Hybridisation
Valence Bond Theory :
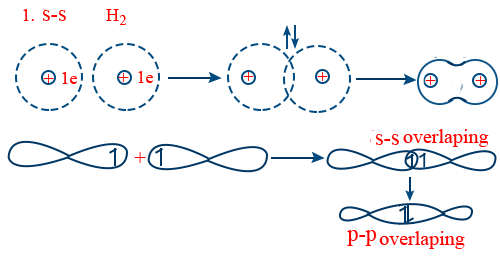
Covalent bond is formed by the overlapping of half filled atomic orbitals.
| σ - bond | π - bond |
| 1. Strong bond | 1. Weak bond |
| 2. Linear overlapping | 2. Side wise overlapping |
| 3. Can exist independently | 3. Can't exist independently |
| 4. Free rotation | 4. no free rotation |
As the size of orbital increases extent of overlapping decreases there by bond strength decreases
H — F > H — Cl > H — Br > H — I
Nuclear charge increases extent of overlap increases
Draw backs :
It failed to explain
- Molecular geometry
- Bond angles
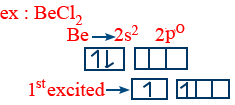
Hybridization :
The intermixing of atomic orbitals of same atom having nearly same energy to produce an equivalent number of hybrid orbitals having identical shapes and energy is called hybridization.
Rules :
→ orbitals undergo hybridization but not electrons
→ Same atom only undergo hybridization
→ Angle is same between two hybrid orbitals
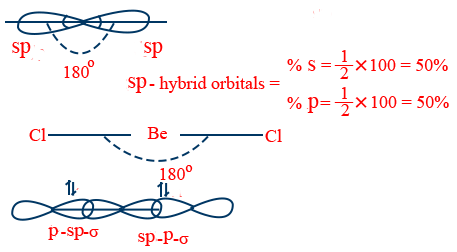
s–p hybridization :
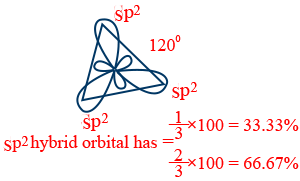
sp2 – hybridization :
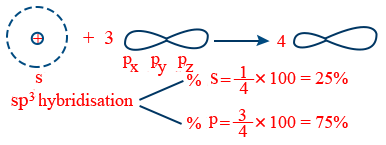
sp3 – hybridization :
Intermixing of 1s and 3p orbitals (px, py, pz)
sp3d – hybridization :
\% \ s \ - \ character - \frac{1}{5} \times 100 = 20 \%
\% \ p \ - \ character - \frac{3}{5} \times 100 = 60 \%
\% \ d \ - \ character - \frac{1}{5} \times 100 = 20 \%
% s character increases electronegativity increases
sp3d2 (or) d2sp3 hybridization :
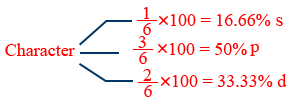
Strong field ligands form d2sp3 or dsp3
Weak field ligands form sp3d2 (or) sp3d hybridization
View the Topic in this Video from 4:58 to 1:00:20
Disclaimer: Compete.etutor.co may from time to time provide links to third party Internet sites under their respective fair use policy and it may from time to time provide materials from such third parties on this website. These third party sites and any third party materials are provided for viewers convenience and for non-commercial educational purpose only. Compete does not operate or control in any respect any information, products or services available on these third party sites. Compete.etutor.co makes no representations whatsoever concerning the content of these sites and the fact that compete.etutor.co has provided a link to such sites is NOT an endorsement, authorization, sponsorship, or affiliation by compete.etutor.co with respect to such sites, its services, the products displayed, its owners, or its providers.

